 |
ARCHIVE # 4: 554 ARTICLES (NOV -SEPT 2006) |
|
|||||
| Visitors Since 03/2006 |
||||||||||||||||||||||||||||||||||||||||
| Click here to read messages from our MySpace Friends HERE'S A FEW OF OUR 1,404 MySpace FRIENDS |
||||||||||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||||||||||

| ||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||
 Click to view 1280 MS Walk photos! | ||||||||||||||||||||||||||||||||||||||||
| "Join a trial at Barrow & receive all medication & study based procedures at no charge!" | ||||||||||||||||||||||||||||||||||||||||
| Stan Swartz, CEO, The MD Health Channel "WE PRODUCED THE FOLLOWING 9 VIDEOS FOR YOU!" Simply click the "video" buttons below: . |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Previious Posts | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| MS NEWS ARCHIVES: by week | ||||||||||||||||||||||||||||||||||||||||
| September 2006 | ||||||||||||||||||||||||||||||||||||||||
| October 2006 | ||||||||||||||||||||||||||||||||||||||||
| November 2006 | ||||||||||||||||||||||||||||||||||||||||
| July 2013 | ||||||||||||||||||||||||||||||||||||||||
| April 2014 | ||||||||||||||||||||||||||||||||||||||||
Tuesday, April 22, 2014
!
WE ARE ADDING THESE TOTALS INTO OUR MAIN TOTALS
Thursday, July 18, 2013
testing!
Tuesday, November 28, 2006
Results of study on prevalence of MS in Lewistown to be presented - Canton Daily Ledger
LEWISTOWN -- Results of a study on the prevalence of multiple sclerosis (MS) in Lewistown and four other small Illinois communities will be announced in a series of presentations at those five towns
Responding to citizen concerns about a perceived high number of MS cases in small Illinois communities, Health Systems Research of the University of Illinois College of Medicine at Rockford applied for a grant to study the prevalence of the disease in DePue, Lewistown, Morrison, Paw Paw and Savanna.
The grant was awarded in October 2002 from the Agency for Toxic Diseases and Disease Registry, a division of the Centers for Disease Control and Prevention. The project was funded as one of five areas of the nation to study MS and ALS (Lou Gehrig's Disease).
The study, which sought to determine local rates and compare them to national levels, has now been completed and the results will be announced at the meetings scheduled in the five small towns in Illinois.
Health Systems Research sought to find all individuals with MS and ALS who lived in the zip code areas of those communities from 1998 to 2002. Self-identifying individuals then completed information on their medical, residential and occupational history and gave permission for their medical records to be reviewed to verify the diagnosis, a press release said.
For more information contact Joel Cowen, principal investigator, at 1-800-854-4461 or joelc@uic.edu.
LEWISTOWN -- Results of a study on the prevalence of multiple sclerosis (MS) in Lewistown and four other small Illinois communities will be announced in a series of presentations at those five towns
Responding to citizen concerns about a perceived high number of MS cases in small Illinois communities, Health Systems Research of the University of Illinois College of Medicine at Rockford applied for a grant to study the prevalence of the disease in DePue, Lewistown, Morrison, Paw Paw and Savanna.
The grant was awarded in October 2002 from the Agency for Toxic Diseases and Disease Registry, a division of the Centers for Disease Control and Prevention. The project was funded as one of five areas of the nation to study MS and ALS (Lou Gehrig's Disease).
The study, which sought to determine local rates and compare them to national levels, has now been completed and the results will be announced at the meetings scheduled in the five small towns in Illinois.
Health Systems Research sought to find all individuals with MS and ALS who lived in the zip code areas of those communities from 1998 to 2002. Self-identifying individuals then completed information on their medical, residential and occupational history and gave permission for their medical records to be reviewed to verify the diagnosis, a press release said.
For more information contact Joel Cowen, principal investigator, at 1-800-854-4461 or joelc@uic.edu.
Antibodies Against Myelin Protein Prominent in Primary Progressive MS
NEW YORK (Reuters Health) Nov 27 - IgG antibodies to the myelin oligodendrocyte glycoprotein (MOG) appear to participate in the more severe type of multiple sclerosis (MS), investigators in Germany report in the PNAS Early Edition, published online on November 27.
Of the self-antigens previously evaluated in MS patients, none has a proven biological activity, senior author Dr. Bernhard Hemmer, from Heinrich Heine University in Dusseldorf, and his associates note.
MOG is present at the outermost surface of the myelin sheath, and anti-MOG antibodies have been implicated in the pathogenesis of MS, making it a "promising target" for further investigation of its role in MS. Up until now, researchers have evaluated fragments or linear constructs of MOG.
To construct a more natural model of MOG, Dr. Hemmer's team transduced human glial cells with full-length human MOG cDNA.
Dr. Hemmer and his associates conclude: "The occurrence of antibodies with demyelinating properties further supports the pathogenic role of the humoral immune system in MS and calls for the development of B cell directed therapies not only for relapsing remitting MS, but also for primary progressive MS.MORE
NEW YORK (Reuters Health) Nov 27 - IgG antibodies to the myelin oligodendrocyte glycoprotein (MOG) appear to participate in the more severe type of multiple sclerosis (MS), investigators in Germany report in the PNAS Early Edition, published online on November 27.
Of the self-antigens previously evaluated in MS patients, none has a proven biological activity, senior author Dr. Bernhard Hemmer, from Heinrich Heine University in Dusseldorf, and his associates note.
MOG is present at the outermost surface of the myelin sheath, and anti-MOG antibodies have been implicated in the pathogenesis of MS, making it a "promising target" for further investigation of its role in MS. Up until now, researchers have evaluated fragments or linear constructs of MOG.
To construct a more natural model of MOG, Dr. Hemmer's team transduced human glial cells with full-length human MOG cDNA.
Dr. Hemmer and his associates conclude: "The occurrence of antibodies with demyelinating properties further supports the pathogenic role of the humoral immune system in MS and calls for the development of B cell directed therapies not only for relapsing remitting MS, but also for primary progressive MS.MORE
Monday, November 27, 2006
WE ARE HAVING AN OVERLOAD ON OUR SERVERS THIS AM
OUR TECHS ARE WORKING ON IT...IT WILL BE FIXED SOON...HOPEFULLY!!
Fatigue and sleep disturbance in multiple sclerosis
Considering the association of sleep disturbance and fatigue in multiple sclerosis (MS), we investigated the presence of sleep disturbances that may be related to fatigue by using objective and subjective measures. We included 27 MS patients with fatigue, 10 MS patients without fatigue and 13 controls. The Pittsburgh sleep quality index score showed significant differences between patient groups and controls. Beck depression inventory scores were significantly higher in fatigued than non-fatigued patients. Comparison of patient groups and controls revealed significant differences for time in bed, sleep efficiency index, sleep continuity index, wake time after sleep onset, total arousal index and periodic limb movement arousal index.
Our study confirms that MS causes sleep fragmentation in terms of both macro and microstructure. Fatigue in MS could be partially explained by disruption of sleep microstructure, poor subjective sleep quality and depression.
Considering the association of sleep disturbance and fatigue in multiple sclerosis (MS), we investigated the presence of sleep disturbances that may be related to fatigue by using objective and subjective measures. We included 27 MS patients with fatigue, 10 MS patients without fatigue and 13 controls. The Pittsburgh sleep quality index score showed significant differences between patient groups and controls. Beck depression inventory scores were significantly higher in fatigued than non-fatigued patients. Comparison of patient groups and controls revealed significant differences for time in bed, sleep efficiency index, sleep continuity index, wake time after sleep onset, total arousal index and periodic limb movement arousal index.
Our study confirms that MS causes sleep fragmentation in terms of both macro and microstructure. Fatigue in MS could be partially explained by disruption of sleep microstructure, poor subjective sleep quality and depression.
Respiratory Muscle Strength Training: Functional Outcomes versus Plasticity
[Department of Communication Sciences and Disorders, University of Florida, Gainesville, FL.]
Respiratory muscle strength training is a paradigm that has been used for numerous years with a variety of populations including but not limited to spinal cord injury, chronic obstructive pulmonary disease, multiple sclerosis, Parkinson's disease, voice disordered, sedentary elderly, and healthy young. The respiratory muscle strength program discussed here is an expiratory muscle strength training and uses a pressure threshold device with a regimented treatment protocol. The primary purpose of the expiratory muscle strength training program is to promote strength in the expiratory muscles. The training protocol occurs five times per day, 5 days a week, and consists of ~15-20 minutes per day of training by the user at home. The device threshold is changed weekly by a clinician to maintain a threshold load of 75% of an individual's maximum expiratory pressure. The threshold setting of the device is always based on the individual's recorded maximum expiratory pressure generated into a digital pressure gauge.
Results of 4 weeks of expiratory muscle strength training protocols indicate up to a 50% improvement for healthy subjects, those with multiple sclerosis, and those with spinal cord injury. The potential transfer of expiratory muscle strength to functional outcomes is discussed, as well as how strength-training paradigms may influence cortical plasticity.
[Department of Communication Sciences and Disorders, University of Florida, Gainesville, FL.]
Respiratory muscle strength training is a paradigm that has been used for numerous years with a variety of populations including but not limited to spinal cord injury, chronic obstructive pulmonary disease, multiple sclerosis, Parkinson's disease, voice disordered, sedentary elderly, and healthy young. The respiratory muscle strength program discussed here is an expiratory muscle strength training and uses a pressure threshold device with a regimented treatment protocol. The primary purpose of the expiratory muscle strength training program is to promote strength in the expiratory muscles. The training protocol occurs five times per day, 5 days a week, and consists of ~15-20 minutes per day of training by the user at home. The device threshold is changed weekly by a clinician to maintain a threshold load of 75% of an individual's maximum expiratory pressure. The threshold setting of the device is always based on the individual's recorded maximum expiratory pressure generated into a digital pressure gauge.
Results of 4 weeks of expiratory muscle strength training protocols indicate up to a 50% improvement for healthy subjects, those with multiple sclerosis, and those with spinal cord injury. The potential transfer of expiratory muscle strength to functional outcomes is discussed, as well as how strength-training paradigms may influence cortical plasticity.
Myelin to Blame for Many Neuropsychiatric Disorders... UCLA Department of Neurology
What makes the human brain unique? Of the many explanations that can be offered, one that doesn't come readily to mind is — myelin.
Conventional wisdom holds that myelin, the sheet of fat that coats a neuron's axon — a long fiber that conducts the neuron's electrical impulses — is akin to the wrapping around an electrical wire, protecting and fostering efficient signaling. But the research of UCLA neurology professor George Bartzokis, M.D., has already shown that myelin problems are implicated in diseases that afflict both young and old — from schizophrenia to Alzheimer's.
Now, in a report published in the journal Biological Psychiatry and available online, Bartzokis argues that the miles of myelin coating in our brain are the key "evolutionary change that defines our uniqueness as a species" and, further, may also be the cause of "our unique vulnerability to highly prevalent neuropsychiatric disorders." The paper argues that viewing the brain as a myelin-dependent "Internet" may be key to developing new and novel treatments against disease and aid in assessing the efficacy of currently available treatments, including the use of nicotine (delivered by a patch, not smoking), which may enhance the growth and maintenance of myelin.
Myelin, argues Bartzokis, who directs the UCLA Memory Disorders and Alzheimer's Disease Clinic, is "a recent invention of evolution. Vertebrates have it; invertebrates don't. And humans have more than any other species."
Bartzokis studied the reported effects of cholinergic treatments, using drugs that are known to improve a neuron's synaptic signaling in people who suffer diseases like Alzheimer's. Furthermore, he notes, some clinical and epidemiological data suggest that such treatments may modify or even delay these diseases.
Looking at such effects from a myelin-centric point of view, Bartzokis argues that cholinergic treatments may have nonsynaptic effects as well, perhaps by enhancing myelination and myelin repair — and the better the myelin, the more efficient the neuron signaling and our "Internet's" function. Specifically, such cholinergic treatments may enhance oligodendrocytes, a type of glia cell in the brain that produces myelin during the brain 's development and constantly maintains and repairs it as we age.
While more work needs to be done to fully understand the role of nonsynaptic cholinergic effects on brain development, said Bartzokis, his hypotheses can easily be tested through in vivo imaging of the brain to study the breakdown and growth of myelin. That will make it possible to directly test in humans the practical utility of the myelin-centered model of the human brain.
Ultimately, it could foster the development of novel treatments, as well as aid in assessing the efficacy of currently available treatments. These include the use of cholinergic treatments that include acetylcholinesterase inhibitors (used to treat Alzheimer's) and nicotine patches.
"Through these rather benign interventions," Bartzokis said, "such effects on the brain's vulnerable oligodendrocyte populations may offer exciting opportunities for the prevention of both developmental and degenerative brain disorders. They deserve much closer scrutiny."MORE: UCLA Department of Neurology
What makes the human brain unique? Of the many explanations that can be offered, one that doesn't come readily to mind is — myelin.
Conventional wisdom holds that myelin, the sheet of fat that coats a neuron's axon — a long fiber that conducts the neuron's electrical impulses — is akin to the wrapping around an electrical wire, protecting and fostering efficient signaling. But the research of UCLA neurology professor George Bartzokis, M.D., has already shown that myelin problems are implicated in diseases that afflict both young and old — from schizophrenia to Alzheimer's.
Now, in a report published in the journal Biological Psychiatry and available online, Bartzokis argues that the miles of myelin coating in our brain are the key "evolutionary change that defines our uniqueness as a species" and, further, may also be the cause of "our unique vulnerability to highly prevalent neuropsychiatric disorders." The paper argues that viewing the brain as a myelin-dependent "Internet" may be key to developing new and novel treatments against disease and aid in assessing the efficacy of currently available treatments, including the use of nicotine (delivered by a patch, not smoking), which may enhance the growth and maintenance of myelin.
Myelin, argues Bartzokis, who directs the UCLA Memory Disorders and Alzheimer's Disease Clinic, is "a recent invention of evolution. Vertebrates have it; invertebrates don't. And humans have more than any other species."
Bartzokis studied the reported effects of cholinergic treatments, using drugs that are known to improve a neuron's synaptic signaling in people who suffer diseases like Alzheimer's. Furthermore, he notes, some clinical and epidemiological data suggest that such treatments may modify or even delay these diseases.
Looking at such effects from a myelin-centric point of view, Bartzokis argues that cholinergic treatments may have nonsynaptic effects as well, perhaps by enhancing myelination and myelin repair — and the better the myelin, the more efficient the neuron signaling and our "Internet's" function. Specifically, such cholinergic treatments may enhance oligodendrocytes, a type of glia cell in the brain that produces myelin during the brain 's development and constantly maintains and repairs it as we age.
While more work needs to be done to fully understand the role of nonsynaptic cholinergic effects on brain development, said Bartzokis, his hypotheses can easily be tested through in vivo imaging of the brain to study the breakdown and growth of myelin. That will make it possible to directly test in humans the practical utility of the myelin-centered model of the human brain.
Ultimately, it could foster the development of novel treatments, as well as aid in assessing the efficacy of currently available treatments. These include the use of cholinergic treatments that include acetylcholinesterase inhibitors (used to treat Alzheimer's) and nicotine patches.
"Through these rather benign interventions," Bartzokis said, "such effects on the brain's vulnerable oligodendrocyte populations may offer exciting opportunities for the prevention of both developmental and degenerative brain disorders. They deserve much closer scrutiny."MORE: UCLA Department of Neurology
Physical therapy arrives: Popularity surges for varied reasons - The Boston Globe
..."Physical therapy is booming. We can't get them out of school fast enough. Hospitals are crying out for physical therapists all over the country," said Dr. Jeffrey B. Palmer , director of physical medicine and rehabilitation at Johns Hopkins Medical Institutions.
Part of the growing demand is because the population is getting older and creakier. But much of it, particularly for problems like back pain, he said, "is the desire for conservative management."
Dr. Lyle Micheli , an orthopedic surgeon and director of sports medicine at Children's Hospital Boston, said he now sends 90 percent of patients "to physical therapy instead of surgery."
At the Spine Center at New England Baptist Hospital, Dr. Geno Martinez, who specializes in rehabilitation medicine, tells many patients that their back pain will improve if they get moving with the help of a physical therapist. Though some physicians still don't believe it, he said, "in reality, back pain, in general, is not a surgical condition."
For those with MS, said Palmer of Hopkins, physical therapy doesn't change the course of the disease, but it can help them move better within their limits.MORE - The Boston Globe
..."Physical therapy is booming. We can't get them out of school fast enough. Hospitals are crying out for physical therapists all over the country," said Dr. Jeffrey B. Palmer , director of physical medicine and rehabilitation at Johns Hopkins Medical Institutions.
Part of the growing demand is because the population is getting older and creakier. But much of it, particularly for problems like back pain, he said, "is the desire for conservative management."
Dr. Lyle Micheli , an orthopedic surgeon and director of sports medicine at Children's Hospital Boston, said he now sends 90 percent of patients "to physical therapy instead of surgery."
At the Spine Center at New England Baptist Hospital, Dr. Geno Martinez, who specializes in rehabilitation medicine, tells many patients that their back pain will improve if they get moving with the help of a physical therapist. Though some physicians still don't believe it, he said, "in reality, back pain, in general, is not a surgical condition."
For those with MS, said Palmer of Hopkins, physical therapy doesn't change the course of the disease, but it can help them move better within their limits.MORE - The Boston Globe
Sunday, November 26, 2006
Linda Beck: Do you believe in miracles? - Salisbury Post
By Linda Beck - Special to the Salisbury Post
"Have you ever felt a miracle taking place in your life? As Christians, we can identify with the miracles that were performed by our Lord and Savoir Jesus Christ during the time that He walked on this earth. He made the blind man able to see, raised Jarius' daughter and Lazarus from the dead and so many more stories of healing.
During my lifetime (soon to be 59 years), I've experienced several healing miracles. Some folks tend to look at the negatives instead of the positives and often ask that question, "Why?" Well, I don't know why I've had Myasthenia Gravis, Multiple Sclerosis, an AVM (arteio venus malformation) in my brain and a series of other health problems. But I do know that according to His Holy Word (the Bible), God does not give us more than we can bear. (His plans for me must have been that I would be able to carry heavy loads.) But there have been times when I thought I couldn't deal with another negative happening in my life and it would be like writing a book to share it all, but I just want to encourage other about the answers to prayer — real modern-day miracles in my life.
There was the period during 1973-1976 when I first experienced the effects of Myasthenia Gravis and was finally diagnosed with this rare progressive muscular weakness disease that had the potential of becoming very disabling. I became familiar with several people who had this disease and have since went to be with the Lord but after several years of ups and downs, it seems my case is now in remission. (Miracle #1)
In 1976, there was the night I nearly died on the operating table. I can remember hearing someone saying, "We're losing her." I also remember my prayers had been that God would heal the staph infection and enable me to return home to finish raising my two daughters. The healing was a long, slow process fraught with many ups and downs but my daughters are now 33 and 38 years old and those prayers were answered. (Miracle #2)
Then in November 1990, I was given a double dose of bad news when I was told I have Multiple Sclerosis, a central nervous system disorder, and an AVM (aterio venus malformation) in my brain which was identified as the cause of the seizures I had experienced in 1989. A miraculous little pill called Dilantin as controlled the seizure activity for 16 years. Praise God for doctors and medicines! (Miracle #3)
Then there were the major Multiple Sclerosis exacerbation in 1992 and the diagnosis of my husband's cancer followed by his death in February 1993. During that extended period of ups and downs, not even the doctors thought I would undergo the healing that led me to driving a car again (after six years of not driving) and being able to walk again, live alone and learn to swim at age 47. No one realized that God was preparing me to be able to travel to speak for Christian Women's Club to tell other women about our Lord and Savior Jesus Christ. (Me, a speaker? - Miracle #4)
Then, in July 2002, I underwent the most catastrophic Multiple Sclerosis attack I have ever experienced. Everything from my breast down was affected and I was told I would never walk again. For 29 days, my toes would not even wiggle and I had to use a transfer board to move from one place to another. Five months later, I was finally able to stand up for the first time after that flare-up. I started using a walker but fell several times so I resigned myself to using a power chair and an electric scooter that a stranger donated to me. (Miracle #5)
Two years later I was finally able to get in the water at the new YMCA by using the electric life. To the amazement of everyone I was able to float and swim again and with the help of the staff there, I have come so far. And that brings us up to the present. Amazing, God's amazing grace! That's what has been taking place. Two days now, I have been able to step out those last two steep steps from the hot tub. (Miracle #6)
This summer the Lord has enabled me to "play in the dirt" in the yard at the house He led me to build in 2002. I have been getting stronger and feeling the best I have since 2002. The buzzing, tingling feelingsMORE - Salisbury Post
By Linda Beck - Special to the Salisbury Post
"Have you ever felt a miracle taking place in your life? As Christians, we can identify with the miracles that were performed by our Lord and Savoir Jesus Christ during the time that He walked on this earth. He made the blind man able to see, raised Jarius' daughter and Lazarus from the dead and so many more stories of healing.
During my lifetime (soon to be 59 years), I've experienced several healing miracles. Some folks tend to look at the negatives instead of the positives and often ask that question, "Why?" Well, I don't know why I've had Myasthenia Gravis, Multiple Sclerosis, an AVM (arteio venus malformation) in my brain and a series of other health problems. But I do know that according to His Holy Word (the Bible), God does not give us more than we can bear. (His plans for me must have been that I would be able to carry heavy loads.) But there have been times when I thought I couldn't deal with another negative happening in my life and it would be like writing a book to share it all, but I just want to encourage other about the answers to prayer — real modern-day miracles in my life.
There was the period during 1973-1976 when I first experienced the effects of Myasthenia Gravis and was finally diagnosed with this rare progressive muscular weakness disease that had the potential of becoming very disabling. I became familiar with several people who had this disease and have since went to be with the Lord but after several years of ups and downs, it seems my case is now in remission. (Miracle #1)
In 1976, there was the night I nearly died on the operating table. I can remember hearing someone saying, "We're losing her." I also remember my prayers had been that God would heal the staph infection and enable me to return home to finish raising my two daughters. The healing was a long, slow process fraught with many ups and downs but my daughters are now 33 and 38 years old and those prayers were answered. (Miracle #2)
Then in November 1990, I was given a double dose of bad news when I was told I have Multiple Sclerosis, a central nervous system disorder, and an AVM (aterio venus malformation) in my brain which was identified as the cause of the seizures I had experienced in 1989. A miraculous little pill called Dilantin as controlled the seizure activity for 16 years. Praise God for doctors and medicines! (Miracle #3)
Then there were the major Multiple Sclerosis exacerbation in 1992 and the diagnosis of my husband's cancer followed by his death in February 1993. During that extended period of ups and downs, not even the doctors thought I would undergo the healing that led me to driving a car again (after six years of not driving) and being able to walk again, live alone and learn to swim at age 47. No one realized that God was preparing me to be able to travel to speak for Christian Women's Club to tell other women about our Lord and Savior Jesus Christ. (Me, a speaker? - Miracle #4)
Then, in July 2002, I underwent the most catastrophic Multiple Sclerosis attack I have ever experienced. Everything from my breast down was affected and I was told I would never walk again. For 29 days, my toes would not even wiggle and I had to use a transfer board to move from one place to another. Five months later, I was finally able to stand up for the first time after that flare-up. I started using a walker but fell several times so I resigned myself to using a power chair and an electric scooter that a stranger donated to me. (Miracle #5)
Two years later I was finally able to get in the water at the new YMCA by using the electric life. To the amazement of everyone I was able to float and swim again and with the help of the staff there, I have come so far. And that brings us up to the present. Amazing, God's amazing grace! That's what has been taking place. Two days now, I have been able to step out those last two steep steps from the hot tub. (Miracle #6)
This summer the Lord has enabled me to "play in the dirt" in the yard at the house He led me to build in 2002. I have been getting stronger and feeling the best I have since 2002. The buzzing, tingling feelingsMORE - Salisbury Post
Woman accepts MS, ready to help others - Wapakoneta Daily News
Sitting on a dark green couch in her Plum Street living room looking through a box of old family photos, a 38-year-old single mother said she does not have the luxury of living in denial about her multiple sclerosis (MS) diagnosis.
“I just can't. I've got my daughter to think about. She's 12,” said Darla McCauley of Wapakoneta. “I'm not going to die of MS. I could die with it, but I'm taking care of myself, getting proper sleep and nutrition. It's all in the attitude, and I've got to survive.
“I've always been a single mother and I want to be there and be able to walk when my daughter graduates from high school,” she added. “I want to show her you can do whatever you want no matter what obstacles you face. I don't want it to interfere.”
McCauley, who suffers severe back and neck pain, face ticks and extreme fatigue from her MS, said she hasn't always been so accepting of the diagnosis she received on Mother's Day 2004MORE - Wapakoneta Daily News
Sitting on a dark green couch in her Plum Street living room looking through a box of old family photos, a 38-year-old single mother said she does not have the luxury of living in denial about her multiple sclerosis (MS) diagnosis.
“I just can't. I've got my daughter to think about. She's 12,” said Darla McCauley of Wapakoneta. “I'm not going to die of MS. I could die with it, but I'm taking care of myself, getting proper sleep and nutrition. It's all in the attitude, and I've got to survive.
“I've always been a single mother and I want to be there and be able to walk when my daughter graduates from high school,” she added. “I want to show her you can do whatever you want no matter what obstacles you face. I don't want it to interfere.”
McCauley, who suffers severe back and neck pain, face ticks and extreme fatigue from her MS, said she hasn't always been so accepting of the diagnosis she received on Mother's Day 2004MORE - Wapakoneta Daily News
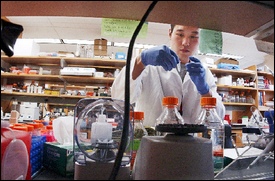
University of Rochester presses quest for stem cell breakthrough - Democrat & Chronicle Newspaper
Pursuit of new disease treatments is building momentum with the work of key researchers
(November 26, 2006) — Quietly but steadily, under the watchful eye of some of the nation's top scientists, hundreds of technicians and researchers isolate cells and scrutinize data in 18 immense laboratories at the University of Rochester Medical Center. They're teasing out the secrets of stem cells, the building blocks of the body, in the hope of finding cures for diseases such as Parkinson's, diabetes and multiple sclerosis.
Some of the work has sparked controversy because it uses days-old human embryos, which some researchers believe can develop into many tissues of the body and in larger quantities than possible with stem cells taken from adult tissue. In turn, discoveries using those cells may lead to many more treatments, for conditions as wide-ranging as spinal cord injuries and blood cancers.
But the issue is not as simple as many political pundits, and some scientists, have led the public to believe. Using stem-cell science to treat people may be close to realization for some conditions but many years away for others. Federal funding for new embryonic work is banned, but private funding is flowing. Using embryos could be vital for some discoveries but completely unnecessary for others.
One UR scientist, neurologist Dr. Steven A. Goldman, recently had a breakthrough, then a setback, in Parkinson's treatment. Yet he might be close to finding a treatment for some neurodegenerative diseases.
Details of the daily work of researchers such as Goldman are largely unknown to the public, if only because the science is so complex and arcane. But shining a spotlight on his lab may improve understanding of the research that could one day change medicine.
"To think, you can actually start to do the things you dream about," Goldman said.
UR expands reach
Directly across from Goldman's desk in the Arthur Kornberg research building next to Strong Memorial Hospital is a dry eraser board filled with scribbling. The notations would probably mean nothing to 99 percent of the population. But for Goldman, it's like reading the back of a cereal box. "It's pretty easy actually," he said.
Goldman is sketching out what is called a progenitor cell, a cell that leads to the formation of the brain's structures. But one has to determine how and why the cell changes to learn how to manipulate it for other purposes. That's where the mapping of the cell comes in, finding the different genes present in each cell that, with the help of molecular signals, cause the cell to turn into its final form.
The cell sketched in black marker on Goldman's board is an astrocyte progenitor, a cell that eventually turns into the star-shaped network of branches and fibers that make up the physical structure of the brain.
Getting his life back, one step at a time | Star-Telegram | 11/26/2006
High-tech device helps a martial artist learn how to walk again
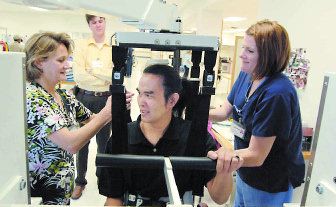
[photo: Physical therapist Debbie Nystrom, left, and rehab technician Beth Peebles position Erwin Villezon in the AutoAmbulator.]

[photo: On a screen, therapists can monitor Villezon's progress on the AutoAmbulator, which simulates walking. "I want to get back to walking the way I once did," he said.]

[photo - Erwin Villezon looks in a mirror while on the AutoAmbulator this month. "It has given him confidence and added normalcy to his life," said his wife, Bobbie Villezon.]
By JAN JARVIS
STAR-TELEGRAM STAFF WRITER
Erwin Villezon pushes himself to walk, even as his right foot drags on the ground.
He makes himself talk despite a tongue that refuses to cooperate. And he forces himself to recall names of people he has no clear memory of.
The same drive that won him movie roles, stuntman jobs and national recognition as a martial artist is now helping him recover from two strokes and tuberculosis in his brain.
As he struggles through physical therapy at the HealthSouth Rehabilitation Hospital of Arlington, Villezon, 46, amazes those who remember how helpless he was a few months ago.
"He would just lay there and sleep; he had no emotions," said Bobbie Villezon, his wife of 26 years. "I've watched him more and more grow back into being my husband."
Three times a week, Erwin Villezon is suspended in a harness that holds him upright over the treadmill. Robotic arms help him move his legs and replicate normal walking patterns.
The AutoAmbulator was developed by HealthSouth Corp. and is being used to rehabilitate people in centers across the country. For people with abnormal gaits caused by spinal injuries, MS and other conditions, the machine may be a path to walking again.
The device simulates walking while supporting the person's weight, said Melissa Higgins, a physical therapist at the HealthSouth facility in south Arlington. It also improves circulation by moving the legs and builds strength through weight-bearing movements.
But it's the psychological effect -- when patients see themselves moving in a mirror -- that often makes the biggest difference.
"People are able to see themselves upright," Higgins said. "Some people have not had the experience of walking in years."
MOTR - Star-Telegram
High-tech device helps a martial artist learn how to walk again

[photo: Physical therapist Debbie Nystrom, left, and rehab technician Beth Peebles position Erwin Villezon in the AutoAmbulator.]

[photo: On a screen, therapists can monitor Villezon's progress on the AutoAmbulator, which simulates walking. "I want to get back to walking the way I once did," he said.]

[photo - Erwin Villezon looks in a mirror while on the AutoAmbulator this month. "It has given him confidence and added normalcy to his life," said his wife, Bobbie Villezon.]
By JAN JARVIS
STAR-TELEGRAM STAFF WRITER
Erwin Villezon pushes himself to walk, even as his right foot drags on the ground.
He makes himself talk despite a tongue that refuses to cooperate. And he forces himself to recall names of people he has no clear memory of.
The same drive that won him movie roles, stuntman jobs and national recognition as a martial artist is now helping him recover from two strokes and tuberculosis in his brain.
As he struggles through physical therapy at the HealthSouth Rehabilitation Hospital of Arlington, Villezon, 46, amazes those who remember how helpless he was a few months ago.
"He would just lay there and sleep; he had no emotions," said Bobbie Villezon, his wife of 26 years. "I've watched him more and more grow back into being my husband."
Three times a week, Erwin Villezon is suspended in a harness that holds him upright over the treadmill. Robotic arms help him move his legs and replicate normal walking patterns.
The AutoAmbulator was developed by HealthSouth Corp. and is being used to rehabilitate people in centers across the country. For people with abnormal gaits caused by spinal injuries, MS and other conditions, the machine may be a path to walking again.
The device simulates walking while supporting the person's weight, said Melissa Higgins, a physical therapist at the HealthSouth facility in south Arlington. It also improves circulation by moving the legs and builds strength through weight-bearing movements.
But it's the psychological effect -- when patients see themselves moving in a mirror -- that often makes the biggest difference.
"People are able to see themselves upright," Higgins said. "Some people have not had the experience of walking in years."
MOTR - Star-Telegram
Saturday, November 25, 2006
FREE BOOKLET DOWNLOAD: "MS Essentials 21: Exercise and physiotherapy: How exercise can help people with MS improve their quality of life" [UK MS Society]
Exercise helps to keep us fit and it does not have to be a chore. That is as much the case for people with multiple sclerosis as anyone else. But what is right for one person who has this very variable, often fluctuating condition may not be right for another.
This new 20-page booklet from the MS Society sets out to help people with MS find the exercise regime which will suit them best and fit into their lives. It begins by explaining how regular exercise can help minimise loss of muscle strength and fitness, unwanted changes in weight and weakened bones.
Different types are described, ranging from aerobics and using small weights to stretching and posture exercises. The booklet also suggests particular exercises for specific MS symptoms, including fatigue, balance, continence problems, anxiety and depression. It describes how physiotherapy can help to improve movement and other function of the body, and how to go about getting the treatment. A section is devoted to exercise for those more seriously affected by MS. CLICK HERE
Exercise helps to keep us fit and it does not have to be a chore. That is as much the case for people with multiple sclerosis as anyone else. But what is right for one person who has this very variable, often fluctuating condition may not be right for another.
This new 20-page booklet from the MS Society sets out to help people with MS find the exercise regime which will suit them best and fit into their lives. It begins by explaining how regular exercise can help minimise loss of muscle strength and fitness, unwanted changes in weight and weakened bones.
Different types are described, ranging from aerobics and using small weights to stretching and posture exercises. The booklet also suggests particular exercises for specific MS symptoms, including fatigue, balance, continence problems, anxiety and depression. It describes how physiotherapy can help to improve movement and other function of the body, and how to go about getting the treatment. A section is devoted to exercise for those more seriously affected by MS. CLICK HERE
Show Your Support: Help Increase Funding for MS: National MS Society
The National Multiple Sclerosis Society has broadcast an Action Alert to increase federal funding for medical research for multiple sclerosis therapies. Anyone touched by MS can show support by signing a petition, which the MS Society is circulating in an effort to collect 200,000 signatures. By providing your signature, you are asking for federal funding to gain a better understanding of multiple sclerosis's causes, leading to more treatments and possibly a cure.CLICK
The National Multiple Sclerosis Society has broadcast an Action Alert to increase federal funding for medical research for multiple sclerosis therapies. Anyone touched by MS can show support by signing a petition, which the MS Society is circulating in an effort to collect 200,000 signatures. By providing your signature, you are asking for federal funding to gain a better understanding of multiple sclerosis's causes, leading to more treatments and possibly a cure.CLICK
PDL BioPharma Announces Roche To Discontinue Co-Development Of Daclizumab
PDL BioPharma, Inc. (Nasdaq: PDLI) announced today that Roche will discontinue its agreement with PDL to jointly develop and commercialize daclizumab for organ transplant patients on longer-term maintenance therapy. Roche made this decision subsequent to a periodic internal review of its development programs. This decision follows another decision by Roche earlier this year to discontinue its involvement in the co-development of daclizumab for the treatment of asthma.
In a separate collaboration, Biogen Idec and PDL are developing daclizumab in multiple sclerosis. "We are evaluating the overall transplant maintenance indication opportunity for daclizumab, while we continue to support the ongoing studies of daclizumab in relapsing/remitting multiple sclerosis, and anticipate results from the ongoing Phase 2 CHOICE study, which is testing daclizumab in combination with beta-interferon, during 2007," said Mark McDade, Chief Executive Officer, PDL BioPharma. MORE
PDL BioPharma, Inc. (Nasdaq: PDLI) announced today that Roche will discontinue its agreement with PDL to jointly develop and commercialize daclizumab for organ transplant patients on longer-term maintenance therapy. Roche made this decision subsequent to a periodic internal review of its development programs. This decision follows another decision by Roche earlier this year to discontinue its involvement in the co-development of daclizumab for the treatment of asthma.
In a separate collaboration, Biogen Idec and PDL are developing daclizumab in multiple sclerosis. "We are evaluating the overall transplant maintenance indication opportunity for daclizumab, while we continue to support the ongoing studies of daclizumab in relapsing/remitting multiple sclerosis, and anticipate results from the ongoing Phase 2 CHOICE study, which is testing daclizumab in combination with beta-interferon, during 2007," said Mark McDade, Chief Executive Officer, PDL BioPharma. MORE
BioMS Medical Raises $: "BioMS Medical Corp ('BioMS' or the 'Corporation') (MS), a leading developer in the treatment of multiple sclerosis (MS), today announced that it has completed a private placement of 6,128,957 million units at a price of CDN $3.41 per unit, for gross proceeds of CDN $20.9 million. It is anticipated that a further closing of approximately 880,000 units for gross proceeds of approximately $3,000,000 may occur within the next two weeks."MORE
Turkey Bowl XXXI -- to fight MS
[Photo: Turkey Bowl organizer Brian Quick, right, talks with his sister, Lisa, at this game. Turkey Bowlers raised more than $5,000 to benefit Lisa, who has multiple sclerosis.]
Brian Quick established the game as a high schooler in 1976. It took on new meaning and status as a fundraiser after Quick's sister, Lisa, was diagnosed with multiple sclerosis a short time later.
Now players must raise at least $50 to get into the three-hour game at Wilde Field outside Lisle Junior High School.
Quick said organizers hope to generate more than $5,000 this year for Lisa and MS research, bringing their grand total since the fundraiser began to more than $115,000.
Six of the 30 players in Friday's Turkey Bowl XXXI were among the original group that got together more than three decades ago.READ MORE
Friday, November 24, 2006
Effectiveness of non-pharmacological interventions for fatigue in adults with multiple sclerosis, rheumatoid arthritis, or systemic lupus erythematosus: a systematic review. -- Medline Abstract
"This paper reports a systematic review of non-pharmacological interventions for fatigue in adults with three common autoimmune conditions. Background. A considerable proportion of people with multiple sclerosis, rheumatoid arthritis, and systemic lupus erythematosus experience compromised quality of life due to fatigue. Recent reviews of pharmacotherapies for fatigue in these conditions remain inconclusive, and systematic evidence for effectiveness of non-pharmacological interventions was unavailable...
Most interventions were tested with people with multiple sclerosis. Exercise, behavioural, nutritional and physiological interventions were associated with statistically significant reductions in fatigue. Aerobic exercise was effective, appropriate and feasible for reducing fatigue among adults with chronic autoimmune conditions. Electromagnetic field devices showed promise. The diversity of interventions, designs, and using 24 different instruments to measure fatigue, limited comparisons. Conclusion. Low impact aerobic exercise gradually increasing in intensity, duration and frequency may be an effective strategy in reducing fatigue in some adults with chronic auto-immune conditionsMOREt.
"This paper reports a systematic review of non-pharmacological interventions for fatigue in adults with three common autoimmune conditions. Background. A considerable proportion of people with multiple sclerosis, rheumatoid arthritis, and systemic lupus erythematosus experience compromised quality of life due to fatigue. Recent reviews of pharmacotherapies for fatigue in these conditions remain inconclusive, and systematic evidence for effectiveness of non-pharmacological interventions was unavailable...
Most interventions were tested with people with multiple sclerosis. Exercise, behavioural, nutritional and physiological interventions were associated with statistically significant reductions in fatigue. Aerobic exercise was effective, appropriate and feasible for reducing fatigue among adults with chronic autoimmune conditions. Electromagnetic field devices showed promise. The diversity of interventions, designs, and using 24 different instruments to measure fatigue, limited comparisons. Conclusion. Low impact aerobic exercise gradually increasing in intensity, duration and frequency may be an effective strategy in reducing fatigue in some adults with chronic auto-immune conditionsMOREt.
MED STUDENT WITH MS...AND INSURANCE PROBLEMS
[Ann Neurol. 2006 Nov 21;60(5):A10-A11 [Epub ahead of print]
"A talented medical student at our institution was recently diagnosed with multiple sclerosis (MS). beta interferon was prescribed; however, her annual cap from student health insurance coverage for outpatient drug expenses is only $3,000, a sum that would be exceeded within 3 months and leave her without coverage for symptomatic medications also required for her care. Unable to pay for the treatment from personal resources, she qualified to receive beta interferon without cost from a universal access program established by the manufacturer. Unfortunately, it was evident within a few months that she had a poor therapeutic response, so her physician prescribed natalizumab. Her health care plan requires that infusion therapy be given at the hospital's infusion center, yet for various reasons, the drug was not yet approved by the formulary. She remains unable to receive the drug"
[Ann Neurol. 2006 Nov 21;60(5):A10-A11 [Epub ahead of print]
"A talented medical student at our institution was recently diagnosed with multiple sclerosis (MS). beta interferon was prescribed; however, her annual cap from student health insurance coverage for outpatient drug expenses is only $3,000, a sum that would be exceeded within 3 months and leave her without coverage for symptomatic medications also required for her care. Unable to pay for the treatment from personal resources, she qualified to receive beta interferon without cost from a universal access program established by the manufacturer. Unfortunately, it was evident within a few months that she had a poor therapeutic response, so her physician prescribed natalizumab. Her health care plan requires that infusion therapy be given at the hospital's infusion center, yet for various reasons, the drug was not yet approved by the formulary. She remains unable to receive the drug"
Laverne and Shirley Star Donates to MS
Michael “Lenny” McKean appeared on Celebrity Jeopardy this month. Michael competed against fellow stars Regis Philbin, Martin Short, Susan Lucci, and Curt Schilling. The winner got to allocate $50,000 toward his/her charity of choice, and each participant is granted $25,000 toward his/her favorite charity just for joining the show. McKean has committed to donating his money to the National Multiple Sclerosis Society in honor of his life-long friend David “Squiggy” Lander. The two are known as the “Lenny and Squiggy” duo from the popular 1970s TV series Laverne and Shirley. Lander was diagnosed with MS in 1999 and has been an active spokesperson for the disease since then. Last fall, McKean generously donated the proceeds from his appearance on Celebrity Jeopardy to the National Multiple Sclerosis Society.
Michael “Lenny” McKean appeared on Celebrity Jeopardy this month. Michael competed against fellow stars Regis Philbin, Martin Short, Susan Lucci, and Curt Schilling. The winner got to allocate $50,000 toward his/her charity of choice, and each participant is granted $25,000 toward his/her favorite charity just for joining the show. McKean has committed to donating his money to the National Multiple Sclerosis Society in honor of his life-long friend David “Squiggy” Lander. The two are known as the “Lenny and Squiggy” duo from the popular 1970s TV series Laverne and Shirley. Lander was diagnosed with MS in 1999 and has been an active spokesperson for the disease since then. Last fall, McKean generously donated the proceeds from his appearance on Celebrity Jeopardy to the National Multiple Sclerosis Society.
CBS 4 News - National MS Health Expo Is November 19th
CBS4’s Maggie Rodriguez is the South Florida spokesperson for the National Multiple Sclerosis Society. She invites you to the Second Annual MS Health Expo this November 19th. The event is taking place at the Signature Grand in Davie, located at 6900 W State Road 84. The expo is from 9am to 2:30pm.MORE
CBS4’s Maggie Rodriguez is the South Florida spokesperson for the National Multiple Sclerosis Society. She invites you to the Second Annual MS Health Expo this November 19th. The event is taking place at the Signature Grand in Davie, located at 6900 W State Road 84. The expo is from 9am to 2:30pm.MORE
thedesertsun.com | ACT for MS shows off strong support at party:
"ACT for MS gave a smashing supporter party at Imago Gallery in Palm Desert.Board president Jim Conway and membership chair Buddy Sklar welcomed guests. All funds raised by this non-profit go directly to local low-income individuals with Multiple Sclerosis."MORE:
"ACT for MS gave a smashing supporter party at Imago Gallery in Palm Desert.Board president Jim Conway and membership chair Buddy Sklar welcomed guests. All funds raised by this non-profit go directly to local low-income individuals with Multiple Sclerosis."MORE:
NEW RRMS DRUG: MBP8298: BioMS Press Release
"Edmonton, Alberta, - BioMS Medical Corp (TSX: MS), a leading developer in the treatment of multiple sclerosis (MS), today announced that the first patients have been enrolled in its placebo controlled multi-center Phase II clinical trial of MBP8298 for the treatment of relapsing remitting multiple sclerosis (RRMS).
“This is a major milestone in our strategy to advance our lead drug into a second indication,” said Mr. Kevin Giese, President of BioMS Medical. “MBP8298 has shown potential to significantly delay disease progression in secondary progressive MS (SPMS) patients with immune response genes HLA-DR2 and/or DR4 and we look forward to evaluating the potential efficacy of our lead drug in RRMS patients, who represent an equally large patient population.”MORE: BioMS Press Release
"Edmonton, Alberta, - BioMS Medical Corp (TSX: MS), a leading developer in the treatment of multiple sclerosis (MS), today announced that the first patients have been enrolled in its placebo controlled multi-center Phase II clinical trial of MBP8298 for the treatment of relapsing remitting multiple sclerosis (RRMS).
“This is a major milestone in our strategy to advance our lead drug into a second indication,” said Mr. Kevin Giese, President of BioMS Medical. “MBP8298 has shown potential to significantly delay disease progression in secondary progressive MS (SPMS) patients with immune response genes HLA-DR2 and/or DR4 and we look forward to evaluating the potential efficacy of our lead drug in RRMS patients, who represent an equally large patient population.”MORE: BioMS Press Release
Men transmit MS more often to their children vs women: THE CARTER EFFECT
[Department of Neurology, Mayo Clinic College of Medicine, Rochester, MN]
OBJECTIVE: Multiple sclerosis (MS) is approximately twice as common among women as men. If men have greater physiologic resistance to MS, they might theoretically require stronger genetic predisposition than women to overcome this resistance. In this circumstance, men would be expected to transmit the disease more often to their children, a phenomenon known as the Carter effect. The authors evaluated whether the Carter effect is present in MS
RESULTS: Fathers with MS transmitted the disease to their children more often (transmitted: 18, not transmitted: 99) than mothers with MS (transmitted: 27, not transmitted: 296) (p = 0.032; OR: 1.99, 95% CI: 1.05, 3.77). Adjusting for both the sex of the affected child and multiple transmissions from a single affected parent, the sex of the affected parent remained as an independent risk factor for transmission of MS to children, fathers transmitting more often than mothers (p = 0.036; OR: 2.21, 95% CI: 1.05, 4.63).
CONCLUSIONS: The authors have demonstrated the Carter effect in multiple sclerosis (MS). These observations may be explained by greater genetic loading in men that leads to relative excess paternal vs maternal transmission. Linkage analysis in genetic studies of MS may be more informative if patrilineal transmission were given additional weighting.
[Department of Neurology, Mayo Clinic College of Medicine, Rochester, MN]
OBJECTIVE: Multiple sclerosis (MS) is approximately twice as common among women as men. If men have greater physiologic resistance to MS, they might theoretically require stronger genetic predisposition than women to overcome this resistance. In this circumstance, men would be expected to transmit the disease more often to their children, a phenomenon known as the Carter effect. The authors evaluated whether the Carter effect is present in MS
RESULTS: Fathers with MS transmitted the disease to their children more often (transmitted: 18, not transmitted: 99) than mothers with MS (transmitted: 27, not transmitted: 296) (p = 0.032; OR: 1.99, 95% CI: 1.05, 3.77). Adjusting for both the sex of the affected child and multiple transmissions from a single affected parent, the sex of the affected parent remained as an independent risk factor for transmission of MS to children, fathers transmitting more often than mothers (p = 0.036; OR: 2.21, 95% CI: 1.05, 4.63).
CONCLUSIONS: The authors have demonstrated the Carter effect in multiple sclerosis (MS). These observations may be explained by greater genetic loading in men that leads to relative excess paternal vs maternal transmission. Linkage analysis in genetic studies of MS may be more informative if patrilineal transmission were given additional weighting.
Thursday, November 23, 2006
Hardships become blessings in the right light, families say | The News Tribune - Tacoma, WA
Trio lends strength to divorced father
I am a divorced father with multiple sclerosis. Some may think I don’t have much to be thankful for. But I have a great kid (who lives with me), a great mom who helps me, and a great friend.
I met him 30 years ago and he’s stuck with me through thick and thin. Imagine, three people who love me. I’m a lucky man.
- STEVE REYNOLDS, Tacoma
Trio lends strength to divorced father
I am a divorced father with multiple sclerosis. Some may think I don’t have much to be thankful for. But I have a great kid (who lives with me), a great mom who helps me, and a great friend.
I met him 30 years ago and he’s stuck with me through thick and thin. Imagine, three people who love me. I’m a lucky man.
- STEVE REYNOLDS, Tacoma
Wednesday, November 22, 2006
SCROLL DOWN TO READ Today's Headlines
Subscribe to our FREE email newsletter!
Let us help you stay up to date on important breaking news about Copaxone, Rebif, Avonex, Novatone, Tysabri, Betaseron...PLUS the 18 new drugs in the pipeline...listed below!
CLICK HERE! Subscribe to our FREE email newsletter...NOW!...PLEASE :-)
PLUS... YOU'LL RECEIVE SPECIAL ALERTS NOTIFYING YOU WHEN THE FOLLOWING 16 NEW MS TREATMENTS BECOME AVAILABLE TO THE PUBLIC:
1...CAMPATH
2...TOVAXIN
3...ORAL FINGOLIMOD - FTY720
4...COPAXONE WITH MITOXANTRONE
5...ORAL LAQUINIMODE BY TEVA
6...ORAL FAMPRIDINE-SR BY SERONO
7...REBIF: NEW FORMULATION
8...ORAL CDP323: Biogen Idec:
9...SATIVEX - CANNABINOID-BASED DRUG
10...MBP8298
11...NICOTINAMIDE: "Daily Nicotinamide Shots
12...NEURODUR.
13...LYRICA: Pfizer's Lyrica(Pregabalin Capsules)
14...SYMADEX
15...Testosterone gel
16...Two CNS Oral Spray Drug Candidates: NovaDel
CLICK HERE! Subscribe to our FREE email newsletter
Headlines on all of 16 treatments listed above...are below:
Subscribe to our FREE email newsletter!
Let us help you stay up to date on important breaking news about Copaxone, Rebif, Avonex, Novatone, Tysabri, Betaseron...PLUS the 18 new drugs in the pipeline...listed below!
CLICK HERE! Subscribe to our FREE email newsletter...NOW!...PLEASE :-)
PLUS... YOU'LL RECEIVE SPECIAL ALERTS NOTIFYING YOU WHEN THE FOLLOWING 16 NEW MS TREATMENTS BECOME AVAILABLE TO THE PUBLIC:
1...CAMPATH
2...TOVAXIN
3...ORAL FINGOLIMOD - FTY720
4...COPAXONE WITH MITOXANTRONE
5...ORAL LAQUINIMODE BY TEVA
6...ORAL FAMPRIDINE-SR BY SERONO
7...REBIF: NEW FORMULATION
8...ORAL CDP323: Biogen Idec:
9...SATIVEX - CANNABINOID-BASED DRUG
10...MBP8298
11...NICOTINAMIDE: "Daily Nicotinamide Shots
12...NEURODUR.
13...LYRICA: Pfizer's Lyrica(Pregabalin Capsules)
14...SYMADEX
15...Testosterone gel
16...Two CNS Oral Spray Drug Candidates: NovaDel
CLICK HERE! Subscribe to our FREE email newsletter
Headlines on all of 16 treatments listed above...are below:
New Therapies in Clinical Trials [Medscape-Med Students November
Fingolimod
MRI results for oral fingolimod (FTY720) were presented from the placebo-controlled, phase II study with active drug extension.[5] At 6 months, the median cumulative number of new and persistent enhancing lesions was 5 for placebo, 1 for fingolimod 1.25 mg (P < .001 vs placebo), and 3 for fingolimod 5 mg (P = .006 vs placebo). The mean number of cumulative enhancing lesions was 14.8 with placebo, 8.4 with fingolimod 1.25 mg, and 5.7 with fingolimod 5 mg. A statistically significant effect on T2 lesions was seen early between months 1 and 2 (P < .003 for both doses). Crossover from placebo to active fingolimod treatment at month 6 resulted in a significant reduction in enhancing and T2 lesions at month 12 on both doses.
BG00012
Oral BG00012, a fumaric acid ester, was studied in 257 patients who were randomized into 4 arms by Kappos and colleagues.[6] The 240-mg thrice-daily dose resulted in a 69% reduction in the total number of enhancing MRI lesions at weeks 12-24 (P < .001). The number of new and enlarging T2-hyperintense lesions with treatment at week 24 was 4.2 ± 5.4 compared with 2.2 ± 5.4 with placebo (P < .001). The number of T1-hypointense lesions was also lower with oral fumarate therapy (1.7 ± 2.5 vs 0.8 ± 2.0; P = .014). A 32% reduction in relapse rate was a trend, but it was not statistically significant.
Cladribine
Oral cladribine is currently being investigated in a phase 3, multicenter international trial for MS. Although the results of this trial are pending, Martinez-Rodriguez and colleagues[7] used the intravenous form of cladribine in 6 patients with aggressive relapsing-remitting disease. These patients were treated with intravenous cladribine 0.07 mg/kg/day for 5 days monthly in 2-4 courses. The EDSS decreased from 5.5 to 7.0 at baseline to 1.5-5.0 at 12 months. The mean annualized relapse rate dropped to 0.71 ± 0.55 from 2.67 ± 0.75 at baseline. After 1 year, cladribine was infused again in 4 patients due to new severe relapses. No significant side effects were seen.
Teriflunomide
O'Connor and colleagues[8] reported on a 144-week open-label extension study of the phase 2, randomized, double-blind, placebo-controlled teriflunomide trial. Teriflunomide demonstrated more than a 61% reduction in the number of combined unique active lesions on MRI compared with placebo over 36 weeks of treatment. In the extension phase, the 55 placebo patients were randomized to receive either 7 mg or 14 mg teriflunomide a day, and a total of 147 patients entered this phase. The placebo patients who switched to 7 mg teriflunomide had a 65% reduction in the number of combined unique active lesions (P = .02). Those placebo patients who switched to 14 mg teriflunomide a day had an 85% reduction in the number of combined unique active lesions (P = .02). Annual relapses were similar between arms at approximately 0.4 relapses per year.
Alemtuzumab
Alemtuzumab is a humanized monoclonal antibody that targets the CD52 antigen and is administered intravenously over 3-5 days annually. In a multicenter, rater-blinded trial of 334 relapsing-remitting patients, patients on alemtuzumab had a 75% reduction in relapse rate (P < .003) and a 60% reduction in the risk for sustained accumulation of disability (P < .05) compared with patients on interferon beta-1a 44 micrograms (mcg) subcutaneously thrice weekly.[9] Four patients developed immune thrombocytopenic purpura from 1.5 to 14 months after their last infusion. One patient died from cerebral hemorrhage after 2 weeks of unrecognized symptoms of immune thrombocytopenic purpura. The other patients were successfully treated with steroids ± rituximab. Compston and colleagues[10] presented thyroid-related findings from the year 2 interim safety analysis. With 2.2 years of median follow-up, 11.1% of patients on alemtuzumab had a thyroid-related clinical adverse event compared with 1.9% of patients on interferon beta-1a. Both hyperthyroid and hypothyroid adverse events were reported, including the development of Graves' disease in 3 patients. Antithyroid-stimulating hormone receptor antibodies and antithyroid peroxidase antibodies without clinical thyroid adverse events were seen in 16.7% of alemtuzumab-treated patients and in 11.3% of interferon-treated patients.
Daclizumab
Daclizumab, a humanized monoclonal antibody, binds to the alpha chain of the interleukin (IL)-2 receptor. Rose and colleagues[11] described results from a phase 1/2 trial of daclizumab in patients with relapsing-remitting MS. Eight patients on interferon therapy were treated with the combination of daclizumab and interferon. One patient developed a severe relapse prior to daclizumab and only received 2 doses at baseline and in 2 weeks. The other 7 patients received daclizumab infusions at 1 mg/kg every 2 weeks for the first month and then monthly for 5.5 months. Five patients were found to have no contrast-enhancing lesions on MRI at 6 months, so interferon was discontinued, and daclizumab was continued at 1.5 mg/kg monthly for a total of 27.5 months of treatment. Two patients had contrast-enhancing lesions at 6 months, so interferon was continued with daclizumab at 1.5 mg/kg monthly. A significant reduction of total contrast-enhancing lesions (P < .05-.001) and new contrast-enhancing lesions (P < .001) was seen compared with pretreatment scans and subsequent scans in 3-month intervals. A significant reduction in relapses also occurred (P < .001).
Anti-CD154 Antibody
Blockage of CD154 prevents costimulation of CD4+ T cells, which disrupt T-cell activation. Kasper and colleagues[12] assessed clinical and MRI progression in subjects with relapsing MS following blockade of CD154. Four cohorts of 3 relapsing MS patients each received 1, 5, 10, or 15 mg/kg of fully humanized monoclonal anti-CD154 antibody every other week for 8 weeks. After 5 years of follow-up, no statistically significant change in disability was found on the EDSS (baseline 2.3 ± 0.5, and 5 years 2.5 ± 1.6; P = .622). Higher doses of anti-CD154 significantly correlated with less disability at 5 years (P < .05). The average annual relapse rate over 5 years was 0.125, whereas the pretreatment rate was 1.0.
Combination Therapy
To assess the benefit of add-on therapy with azathioprine and prednisone, a randomized, double-blind, placebo-controlled study of 181 relapsing patients on intramuscular weekly interferon beta-1a was conducted.[13] Over 2 years, the annualized relapse rate was 1.19 for patients only on interferon. The relapse rate was 1.06 for those on azathioprine 50 mg daily and interferon. Lastly, the relapse rate was 0.80 for patients on azathioprine, interferon, and prednisone 10 mg every other day. The differences in the relapse rate and in the cumulative proportion of patients with sustained disability progression at 2 years were not significant. Triple-combination therapy was superior to monotherapy with interferon on T2 lesion volume (P = .015). Combination therapy was safe and well tolerated with similar rates of infection among the groups. Perhaps greater efficacy would have been derived had the investigators used higher doses of azathioprine.
Induction with mitoxantrone prior to glatiramer therapy was investigated in a randomized trial of 40 relapsing-remitting patients with at least 1 gadolinium-enhancing lesion at baseline.[14] The patients received either daily glatiramer acetate 20 mg subcutaneously for 15 months or monthly intravenous mitoxantrone for 3 months and then glatiramer acetate for 12 months. Mitoxantrone induction produced an 89% greater reduction in enhancing lesions at 9 months (P = .0001) than glatiramer alone. Patients who received glatiramer alone had a 47% reduction in enhancing lesion frequency at 9 months and an 87% reduction at 15 months. The relapse rate over 15 months was 0.16 for the mitoxantrone induction group and 0.32 for the glatiramer-only group. The relapse rates over 24 months were 0.24 for patients who received glatiramer acetate after mitoxantrone induction and 0.62 for patients who received glatiramer acetate alone. The reduction in relapse rate was a trend in favor of the combination therapy, but the trial design does not allow one to determine whether this result is superior to that observed by administering mitoxantrone alone.
Updates on Current Therapies
New Interferon beta-1a Formulation
A new formulation of subcutaneous interferon beta-1a without human serum albumin was studied in 260 patients in a single-arm, open-label, multicenter trial.[15] At 48 weeks, neutralizing antibodies greater then 20 neutralizing units/mL were detected in 13.9% of patients compared with 24.4% in the Evidence for Interferon Dose Response: European-North American Comparative Efficacy (EVIDENCE) trial. Persistently positive antibody incidence at 48 weeks was 2.5% compared with 14.3% in the EVIDENCE trial. The incidence of injection site reactions was 29.6% with the new formulation compared with 83.3% in the EVIDENCE trial. Flulike symptoms were higher at 70.8% compared with 48.1% in the EVIDENCE trial. Only 38% of patients who received the new formulation were taking anti-inflammatory medications or anilides at study day 1, which likely contributed to the higher incidence of flu symptoms. The lower incidence of neutralizing antibodies may be potentially clinically beneficial. The study is ongoing to assess the persistently positive neutralizing antibody incidence at 96 weeks because these antibodies generally form over the first 18 months of therapy.
Glatiramer Acetate
Double-dose (40 mg) glatiramer acetate was compared with the standard 20-mg dose by Cohen and colleagues[16] in a randomized, double-blind trial. Of the 229 patients screened, only 90 had 1-15 enhancing MRI lesions at baseline and were randomized to receive 20 or 40 mg of daily subcutaneous glatiramer acetate. The higher dose resulted in a 38% reduction of total enhancing lesions over months 7-9, but this primary endpoint was not statistically significant (P = .09). Seventy-six percent of patients on the 40-mg dose and 52% on the 20-mg dose were relapse-free, which was a significant benefit. There was a trend in favor of the higher dose in the relapse rate; the rate was 0.43 with 40 mg and 0.57 with 20 mg (P = .12). The rates of immediate postinjection reactions were 22.7% with 20 mg and 32.6% with 40 mg. Further study of higher dose glatiramer is being pursued in a phase 3 clinical trial.
Interferon beta-1b
Goodin and colleagues[17] examined the rates of neutralizing antibody-positive titers in 2 cohorts of patients with a poor clinical response to interferon beta-1b therapy and 1 cohort of patients unselected for response to therapy. This analysis of 6698 patients was conducted to gain an understanding of the clinical impact of these antibodies. Out of the 1998 patients in the North American cohort, 94% had neutralizing antibody testing for disease worsening. Sixty-seven percent had disease progression for at least 6 months, and 34% had at least 3 exacerbations that required steroids and/or hospitalizations per year. Of interest, only 21.3% of patients in the North American cohort were neutralizing antibody-positive with a mean duration of interferon treatment of 3.32 years. Compulsory testing was performed in the Australian cohort of 2271 patients regardless of their clinical response. If neutralizing antibody-positive status was a principal cause for worsening disease, the incidence of positive neutralizing antibodies would be expected to be lower in the Australian cohort with routine testing than in the North American cohort with testing for worsening disease. However, in the Australian cohort, 37% of patients had at least 20 neutralizing units/mL of neutralizing antibodies. Therefore, these results suggest that antibody positivity does not appear to be the major etiology for worsening MS.
Natalizumab
O'Connor and colleagues[18] presented the pivotal trial extension results after natalizumab dosing was suspended. The annualized relapse rate for 1866 patients increased monthly after treatment cessation and peaked at 0.64 at 7 months. Data from 341 patients who had MRI results greater than 60 days after natalizumab discontinuation also experienced a rise in enhancing lesions over 6 months. No new cases of progressive multifocal leukoencephalopathy were reported.
Mitoxantrone
Le Page and colleagues[19] presented the long-term safety data for mitoxantrone in a French cohort of 802 patients. All patients had at least 5 years of follow-up. One patient developed acute congestive heart failure. Thirty-nine patients (4.9%) developed an asymptomatic reduction of their left ventricular ejection fraction below 50%, but it was only transitory in 26 patients. Two patients (0.25%) developed therapy-related acute myeloblastic leukemia 20 and 22 months after the initiation of treatment. One of the 2 patients died regardless of receiving specific chemotherapy. Persistent amenorrhea occurred in 5.4% of women less than 35 and in 31% of women 35 and older. With up to 15 years of follow-up, 51 patients had died but only the leukemia patient's death was considered treatment-related. Thirty-three patients' deaths were considered complications of severe MS.
Therapeutic Development in Experimental Models
IL-17
IL-17, a proinflammatory cytokine, can be blocked with a monoclonal antibody. Smith and colleagues[20] tested the hypothesis that IL-17 was responsible for driving relapses in the spontaneous chronic-relapsing experimental allergic encephalomyelitis model in the mouse, with an antimouse IL-17 monoclonal immunoglobulin antibody. Ten milligrams per kilogram of anti-IL-17 antibody were administered weekly subcutaneously during remission. The anti-IL-17 antibody significantly reduced the relapse incidence and the inhibited neurologic deficit formation. Treatment with the antibody prior to the acute phase delayed disease, but failed to reduce the clinical score. Because IL-17 may be playing an important role in driving relapses, anti-IL-17 therapies may prove to be effective treatment options for relapsing-remitting disease.
c-Jun N-terminal Kinase Inhibition
The c-Jun N-terminal kinase (JNK) pathway, which can be induced in activated T cells, affects gene expression, cellular survival, and cellular proliferation in response to cytokines. These events are associated with the pathogenesis of autoimmune diseases, such as MS. Ferrandi and colleagues[21] investigated the potential role of the JNK pathway in MS. The JNK2 isoform was upregulated in peripheral blood mononuclear cells of patients with relapsing-remitting disease. In vitro administration of a JNK inhibitor resulted in a significant reduction in cell proliferation with triggering of T-cell apoptosis and c-Jun dephosphorylation. The JNK inhibitor, given in daily oral doses, reduced the severity of pathology and delayed the onset of disease in experimental allergic encephalomyelitis. These results support the hypothesis that the inhibition of the JNK pathway could have a role in the treatment of relapsing-remitting MS.
Tyrosine Kinase Inhibition
C-1311 (Symadex), a tyrosine kinase inhibitor, disrupts trafficking of autoreactive cells and concomitant angiogenic processes. Karlik and Ajami[22] presented results showing that treatment initiated in the chronic phase of guinea pig experimental allergic encephalomyelitis showed reversal of clinical and pathologic signs. Treatment with C-1311 was associated with remyelination and modulation of vascular changes.
Arundic Acid
Arundic acid (ONO-2506) is a compound that modulates the function of astrocytes. Studies are examining its use in stroke, Parkinson's disease, and Alzheimer's disease. Arundic acid may enhance the uptake of the neurotransmitter glutamate by activation of astrocytic glutamate transporter receptors in ischemia models. With less extracellular glutamate, neurons are more protected from glutamate influx and cell death. Arundic acid was studied by Takizawa and colleagues[23] in chronic progressive and relapsing-remitting experimental allergic encephalomyelitis. Treatment resulted in milder neurologic symptoms and fewer demyelinating lesions in the brain and spinal cord.
Sphingosine-1-phosphate Receptors and Extracellular Receptor Regulated Kinase Phosphorylation
FTY-720 (fingolimod) also may modulate astrocyte function, which could be beneficial in MS. FTY-720 can activate subtypes 1 and 3 of sphingosine-1-phosphate (S1P) receptors on astrocytes. Osinde and Dev[24] determined which receptor subtype is involved in extracellular receptor regulated kinase (ERK) phosphorylation in astrocytes. Activation of these receptors was measured with downstream signaling via adenylyl cyclase, phospholipase C, and ERK. The study authors found that the S1P receptor subtype-1 mediates ERK phosphorylation in astrocytes. Potential astrocytic functions that are beneficial in MS are those that promote neuronal survival and remyelination and strengthen the contact sites between endothelial cells at the blood-brain barrier.
Fingolimod
MRI results for oral fingolimod (FTY720) were presented from the placebo-controlled, phase II study with active drug extension.[5] At 6 months, the median cumulative number of new and persistent enhancing lesions was 5 for placebo, 1 for fingolimod 1.25 mg (P < .001 vs placebo), and 3 for fingolimod 5 mg (P = .006 vs placebo). The mean number of cumulative enhancing lesions was 14.8 with placebo, 8.4 with fingolimod 1.25 mg, and 5.7 with fingolimod 5 mg. A statistically significant effect on T2 lesions was seen early between months 1 and 2 (P < .003 for both doses). Crossover from placebo to active fingolimod treatment at month 6 resulted in a significant reduction in enhancing and T2 lesions at month 12 on both doses.
BG00012
Oral BG00012, a fumaric acid ester, was studied in 257 patients who were randomized into 4 arms by Kappos and colleagues.[6] The 240-mg thrice-daily dose resulted in a 69% reduction in the total number of enhancing MRI lesions at weeks 12-24 (P < .001). The number of new and enlarging T2-hyperintense lesions with treatment at week 24 was 4.2 ± 5.4 compared with 2.2 ± 5.4 with placebo (P < .001). The number of T1-hypointense lesions was also lower with oral fumarate therapy (1.7 ± 2.5 vs 0.8 ± 2.0; P = .014). A 32% reduction in relapse rate was a trend, but it was not statistically significant.
Cladribine
Oral cladribine is currently being investigated in a phase 3, multicenter international trial for MS. Although the results of this trial are pending, Martinez-Rodriguez and colleagues[7] used the intravenous form of cladribine in 6 patients with aggressive relapsing-remitting disease. These patients were treated with intravenous cladribine 0.07 mg/kg/day for 5 days monthly in 2-4 courses. The EDSS decreased from 5.5 to 7.0 at baseline to 1.5-5.0 at 12 months. The mean annualized relapse rate dropped to 0.71 ± 0.55 from 2.67 ± 0.75 at baseline. After 1 year, cladribine was infused again in 4 patients due to new severe relapses. No significant side effects were seen.
Teriflunomide
O'Connor and colleagues[8] reported on a 144-week open-label extension study of the phase 2, randomized, double-blind, placebo-controlled teriflunomide trial. Teriflunomide demonstrated more than a 61% reduction in the number of combined unique active lesions on MRI compared with placebo over 36 weeks of treatment. In the extension phase, the 55 placebo patients were randomized to receive either 7 mg or 14 mg teriflunomide a day, and a total of 147 patients entered this phase. The placebo patients who switched to 7 mg teriflunomide had a 65% reduction in the number of combined unique active lesions (P = .02). Those placebo patients who switched to 14 mg teriflunomide a day had an 85% reduction in the number of combined unique active lesions (P = .02). Annual relapses were similar between arms at approximately 0.4 relapses per year.
Alemtuzumab
Alemtuzumab is a humanized monoclonal antibody that targets the CD52 antigen and is administered intravenously over 3-5 days annually. In a multicenter, rater-blinded trial of 334 relapsing-remitting patients, patients on alemtuzumab had a 75% reduction in relapse rate (P < .003) and a 60% reduction in the risk for sustained accumulation of disability (P < .05) compared with patients on interferon beta-1a 44 micrograms (mcg) subcutaneously thrice weekly.[9] Four patients developed immune thrombocytopenic purpura from 1.5 to 14 months after their last infusion. One patient died from cerebral hemorrhage after 2 weeks of unrecognized symptoms of immune thrombocytopenic purpura. The other patients were successfully treated with steroids ± rituximab. Compston and colleagues[10] presented thyroid-related findings from the year 2 interim safety analysis. With 2.2 years of median follow-up, 11.1% of patients on alemtuzumab had a thyroid-related clinical adverse event compared with 1.9% of patients on interferon beta-1a. Both hyperthyroid and hypothyroid adverse events were reported, including the development of Graves' disease in 3 patients. Antithyroid-stimulating hormone receptor antibodies and antithyroid peroxidase antibodies without clinical thyroid adverse events were seen in 16.7% of alemtuzumab-treated patients and in 11.3% of interferon-treated patients.
Daclizumab
Daclizumab, a humanized monoclonal antibody, binds to the alpha chain of the interleukin (IL)-2 receptor. Rose and colleagues[11] described results from a phase 1/2 trial of daclizumab in patients with relapsing-remitting MS. Eight patients on interferon therapy were treated with the combination of daclizumab and interferon. One patient developed a severe relapse prior to daclizumab and only received 2 doses at baseline and in 2 weeks. The other 7 patients received daclizumab infusions at 1 mg/kg every 2 weeks for the first month and then monthly for 5.5 months. Five patients were found to have no contrast-enhancing lesions on MRI at 6 months, so interferon was discontinued, and daclizumab was continued at 1.5 mg/kg monthly for a total of 27.5 months of treatment. Two patients had contrast-enhancing lesions at 6 months, so interferon was continued with daclizumab at 1.5 mg/kg monthly. A significant reduction of total contrast-enhancing lesions (P < .05-.001) and new contrast-enhancing lesions (P < .001) was seen compared with pretreatment scans and subsequent scans in 3-month intervals. A significant reduction in relapses also occurred (P < .001).
Anti-CD154 Antibody
Blockage of CD154 prevents costimulation of CD4+ T cells, which disrupt T-cell activation. Kasper and colleagues[12] assessed clinical and MRI progression in subjects with relapsing MS following blockade of CD154. Four cohorts of 3 relapsing MS patients each received 1, 5, 10, or 15 mg/kg of fully humanized monoclonal anti-CD154 antibody every other week for 8 weeks. After 5 years of follow-up, no statistically significant change in disability was found on the EDSS (baseline 2.3 ± 0.5, and 5 years 2.5 ± 1.6; P = .622). Higher doses of anti-CD154 significantly correlated with less disability at 5 years (P < .05). The average annual relapse rate over 5 years was 0.125, whereas the pretreatment rate was 1.0.
Combination Therapy
To assess the benefit of add-on therapy with azathioprine and prednisone, a randomized, double-blind, placebo-controlled study of 181 relapsing patients on intramuscular weekly interferon beta-1a was conducted.[13] Over 2 years, the annualized relapse rate was 1.19 for patients only on interferon. The relapse rate was 1.06 for those on azathioprine 50 mg daily and interferon. Lastly, the relapse rate was 0.80 for patients on azathioprine, interferon, and prednisone 10 mg every other day. The differences in the relapse rate and in the cumulative proportion of patients with sustained disability progression at 2 years were not significant. Triple-combination therapy was superior to monotherapy with interferon on T2 lesion volume (P = .015). Combination therapy was safe and well tolerated with similar rates of infection among the groups. Perhaps greater efficacy would have been derived had the investigators used higher doses of azathioprine.
Induction with mitoxantrone prior to glatiramer therapy was investigated in a randomized trial of 40 relapsing-remitting patients with at least 1 gadolinium-enhancing lesion at baseline.[14] The patients received either daily glatiramer acetate 20 mg subcutaneously for 15 months or monthly intravenous mitoxantrone for 3 months and then glatiramer acetate for 12 months. Mitoxantrone induction produced an 89% greater reduction in enhancing lesions at 9 months (P = .0001) than glatiramer alone. Patients who received glatiramer alone had a 47% reduction in enhancing lesion frequency at 9 months and an 87% reduction at 15 months. The relapse rate over 15 months was 0.16 for the mitoxantrone induction group and 0.32 for the glatiramer-only group. The relapse rates over 24 months were 0.24 for patients who received glatiramer acetate after mitoxantrone induction and 0.62 for patients who received glatiramer acetate alone. The reduction in relapse rate was a trend in favor of the combination therapy, but the trial design does not allow one to determine whether this result is superior to that observed by administering mitoxantrone alone.
Updates on Current Therapies
New Interferon beta-1a Formulation
A new formulation of subcutaneous interferon beta-1a without human serum albumin was studied in 260 patients in a single-arm, open-label, multicenter trial.[15] At 48 weeks, neutralizing antibodies greater then 20 neutralizing units/mL were detected in 13.9% of patients compared with 24.4% in the Evidence for Interferon Dose Response: European-North American Comparative Efficacy (EVIDENCE) trial. Persistently positive antibody incidence at 48 weeks was 2.5% compared with 14.3% in the EVIDENCE trial. The incidence of injection site reactions was 29.6% with the new formulation compared with 83.3% in the EVIDENCE trial. Flulike symptoms were higher at 70.8% compared with 48.1% in the EVIDENCE trial. Only 38% of patients who received the new formulation were taking anti-inflammatory medications or anilides at study day 1, which likely contributed to the higher incidence of flu symptoms. The lower incidence of neutralizing antibodies may be potentially clinically beneficial. The study is ongoing to assess the persistently positive neutralizing antibody incidence at 96 weeks because these antibodies generally form over the first 18 months of therapy.
Glatiramer Acetate
Double-dose (40 mg) glatiramer acetate was compared with the standard 20-mg dose by Cohen and colleagues[16] in a randomized, double-blind trial. Of the 229 patients screened, only 90 had 1-15 enhancing MRI lesions at baseline and were randomized to receive 20 or 40 mg of daily subcutaneous glatiramer acetate. The higher dose resulted in a 38% reduction of total enhancing lesions over months 7-9, but this primary endpoint was not statistically significant (P = .09). Seventy-six percent of patients on the 40-mg dose and 52% on the 20-mg dose were relapse-free, which was a significant benefit. There was a trend in favor of the higher dose in the relapse rate; the rate was 0.43 with 40 mg and 0.57 with 20 mg (P = .12). The rates of immediate postinjection reactions were 22.7% with 20 mg and 32.6% with 40 mg. Further study of higher dose glatiramer is being pursued in a phase 3 clinical trial.
Interferon beta-1b
Goodin and colleagues[17] examined the rates of neutralizing antibody-positive titers in 2 cohorts of patients with a poor clinical response to interferon beta-1b therapy and 1 cohort of patients unselected for response to therapy. This analysis of 6698 patients was conducted to gain an understanding of the clinical impact of these antibodies. Out of the 1998 patients in the North American cohort, 94% had neutralizing antibody testing for disease worsening. Sixty-seven percent had disease progression for at least 6 months, and 34% had at least 3 exacerbations that required steroids and/or hospitalizations per year. Of interest, only 21.3% of patients in the North American cohort were neutralizing antibody-positive with a mean duration of interferon treatment of 3.32 years. Compulsory testing was performed in the Australian cohort of 2271 patients regardless of their clinical response. If neutralizing antibody-positive status was a principal cause for worsening disease, the incidence of positive neutralizing antibodies would be expected to be lower in the Australian cohort with routine testing than in the North American cohort with testing for worsening disease. However, in the Australian cohort, 37% of patients had at least 20 neutralizing units/mL of neutralizing antibodies. Therefore, these results suggest that antibody positivity does not appear to be the major etiology for worsening MS.
Natalizumab
O'Connor and colleagues[18] presented the pivotal trial extension results after natalizumab dosing was suspended. The annualized relapse rate for 1866 patients increased monthly after treatment cessation and peaked at 0.64 at 7 months. Data from 341 patients who had MRI results greater than 60 days after natalizumab discontinuation also experienced a rise in enhancing lesions over 6 months. No new cases of progressive multifocal leukoencephalopathy were reported.
Mitoxantrone
Le Page and colleagues[19] presented the long-term safety data for mitoxantrone in a French cohort of 802 patients. All patients had at least 5 years of follow-up. One patient developed acute congestive heart failure. Thirty-nine patients (4.9%) developed an asymptomatic reduction of their left ventricular ejection fraction below 50%, but it was only transitory in 26 patients. Two patients (0.25%) developed therapy-related acute myeloblastic leukemia 20 and 22 months after the initiation of treatment. One of the 2 patients died regardless of receiving specific chemotherapy. Persistent amenorrhea occurred in 5.4% of women less than 35 and in 31% of women 35 and older. With up to 15 years of follow-up, 51 patients had died but only the leukemia patient's death was considered treatment-related. Thirty-three patients' deaths were considered complications of severe MS.
Therapeutic Development in Experimental Models
IL-17
IL-17, a proinflammatory cytokine, can be blocked with a monoclonal antibody. Smith and colleagues[20] tested the hypothesis that IL-17 was responsible for driving relapses in the spontaneous chronic-relapsing experimental allergic encephalomyelitis model in the mouse, with an antimouse IL-17 monoclonal immunoglobulin antibody. Ten milligrams per kilogram of anti-IL-17 antibody were administered weekly subcutaneously during remission. The anti-IL-17 antibody significantly reduced the relapse incidence and the inhibited neurologic deficit formation. Treatment with the antibody prior to the acute phase delayed disease, but failed to reduce the clinical score. Because IL-17 may be playing an important role in driving relapses, anti-IL-17 therapies may prove to be effective treatment options for relapsing-remitting disease.
c-Jun N-terminal Kinase Inhibition
The c-Jun N-terminal kinase (JNK) pathway, which can be induced in activated T cells, affects gene expression, cellular survival, and cellular proliferation in response to cytokines. These events are associated with the pathogenesis of autoimmune diseases, such as MS. Ferrandi and colleagues[21] investigated the potential role of the JNK pathway in MS. The JNK2 isoform was upregulated in peripheral blood mononuclear cells of patients with relapsing-remitting disease. In vitro administration of a JNK inhibitor resulted in a significant reduction in cell proliferation with triggering of T-cell apoptosis and c-Jun dephosphorylation. The JNK inhibitor, given in daily oral doses, reduced the severity of pathology and delayed the onset of disease in experimental allergic encephalomyelitis. These results support the hypothesis that the inhibition of the JNK pathway could have a role in the treatment of relapsing-remitting MS.
Tyrosine Kinase Inhibition
C-1311 (Symadex), a tyrosine kinase inhibitor, disrupts trafficking of autoreactive cells and concomitant angiogenic processes. Karlik and Ajami[22] presented results showing that treatment initiated in the chronic phase of guinea pig experimental allergic encephalomyelitis showed reversal of clinical and pathologic signs. Treatment with C-1311 was associated with remyelination and modulation of vascular changes.
Arundic Acid
Arundic acid (ONO-2506) is a compound that modulates the function of astrocytes. Studies are examining its use in stroke, Parkinson's disease, and Alzheimer's disease. Arundic acid may enhance the uptake of the neurotransmitter glutamate by activation of astrocytic glutamate transporter receptors in ischemia models. With less extracellular glutamate, neurons are more protected from glutamate influx and cell death. Arundic acid was studied by Takizawa and colleagues[23] in chronic progressive and relapsing-remitting experimental allergic encephalomyelitis. Treatment resulted in milder neurologic symptoms and fewer demyelinating lesions in the brain and spinal cord.
Sphingosine-1-phosphate Receptors and Extracellular Receptor Regulated Kinase Phosphorylation
FTY-720 (fingolimod) also may modulate astrocyte function, which could be beneficial in MS. FTY-720 can activate subtypes 1 and 3 of sphingosine-1-phosphate (S1P) receptors on astrocytes. Osinde and Dev[24] determined which receptor subtype is involved in extracellular receptor regulated kinase (ERK) phosphorylation in astrocytes. Activation of these receptors was measured with downstream signaling via adenylyl cyclase, phospholipase C, and ERK. The study authors found that the S1P receptor subtype-1 mediates ERK phosphorylation in astrocytes. Potential astrocytic functions that are beneficial in MS are those that promote neuronal survival and remyelination and strengthen the contact sites between endothelial cells at the blood-brain barrier.
ECTRIMS 2006 - New Advances in Clinical Trial Design [MedScape for Med Students}
Advances in clinical trial design and in the treatment of multiple sclerosis (MS) were presented during the 22nd Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), September 27-30, 2006, in Madrid, Spain. The highlights of new findings are reviewed, and implications for clinical practice are discussed.
Clinical Trial Design
Is it ethical to continue to have placebo arms in MS treatment clinical trials? Because therapies exist for relapsing-remitting and secondary progressive MS, randomizing patients to placebo for 2 years has become more controversial. The placebo arm has been critical in measuring the placebo effect. Relapse rates of the placebo group can be reduced by 30% to 50% as patients transition from prestudy to the active placebo phase. Without information of patients on placebo, treatment effects would be overestimated.[1]
Options to avoid a placebo trial include designs that employ add-on therapy, an external placebo group, and an active comparator. The Safety and Efficacy of Natalizumab in Combination with Avonex (interferon beta-1a) (SENTINEL) trial is an example of add-on therapy because natalizumab was added to interferon beta-1a intramuscularly in half of the trial patients. With an external placebo group, such as the Sylvia Lawry data set, all patients can also be on active treatment in a clinical trial. However, historical control groups can have very different outcomes with the same inclusion criteria. For example, patients in different secondary progressive trials of interferon beta-1b had very different disease progression results, although patients were selected with the same inclusion criteria. Active comparator trials are another option in which one treatment is compared with an existing treatment.[2] For example, the Betaferon Efficacy Yielding Outcomes of a New Dose (BEYOND) trial compares 2 doses of interferon beta-1b with glatiramer.
Criteria to ethically perform placebo trials in MS patients must start with informed consent, including the option of being randomized to not receive treatment. Patients should not have tolerated, failed, or refused current treatments. The trial design should be of short duration and have surrogate markers. Rules for patients to drop out of the trial for worsening disease should be clearly delineated. Equipoise is paramount in which the investigator is uncertain of the outcomes before placing patients on placebo.[1]
An important outcomes measure of the pivotal phase 3 MS trials has been confirmed progression of disability. Patients are considered to have confirmed progression with a documented Extended Disability Status Scale (EDSS) increase of greater than 1.0 point on 2 examinations 3-6 months apart. Butzkueven and colleagues[3] assessed whether the trial definition of "confirmed disability progression" equates with "long-term sustained" EDSS progression with the prospectively acquired global MSBase dataset. In their analysis of 4043 relapsing-remitting and secondary progressive patients from 33 contributing MS clinics, 981 had confirmed progression of disability on neurologic exams at least 3 months apart. After 2 years of confirmed disability progression in relapsing-remitting disease, 25% of patients' EDSS scores returned to baseline. After 5 years, 50% of relapsing-remitting patients with confirmed progression returned to baseline level of disability. Fifteen percent of patients with secondary progressive disease returned to baseline level of disability.
Early evaluation of new treatments frequently relies on MRI endpoints. Many trials, such as the 20-mg vs 40-mg glatiramer acetate phase 2 trial, have screened patients for enhancing lesions to assist in determining efficacy with fewer patients. Zhao and colleagues[4] examined the short-term changes in monthly gadolinium-enhancing lesion activity in patients with different initial activity levels. In the 65 placebo patients studied, 49% had no enhancing lesions at baseline. Of this group, 75% developed new lesions over 9 months. All patients with enhancing lesions at baseline had at least 2 more enhancing lesions over 9 months with monthly MRI scans.
Advances in clinical trial design and in the treatment of multiple sclerosis (MS) were presented during the 22nd Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), September 27-30, 2006, in Madrid, Spain. The highlights of new findings are reviewed, and implications for clinical practice are discussed.
Clinical Trial Design
Is it ethical to continue to have placebo arms in MS treatment clinical trials? Because therapies exist for relapsing-remitting and secondary progressive MS, randomizing patients to placebo for 2 years has become more controversial. The placebo arm has been critical in measuring the placebo effect. Relapse rates of the placebo group can be reduced by 30% to 50% as patients transition from prestudy to the active placebo phase. Without information of patients on placebo, treatment effects would be overestimated.[1]
Options to avoid a placebo trial include designs that employ add-on therapy, an external placebo group, and an active comparator. The Safety and Efficacy of Natalizumab in Combination with Avonex (interferon beta-1a) (SENTINEL) trial is an example of add-on therapy because natalizumab was added to interferon beta-1a intramuscularly in half of the trial patients. With an external placebo group, such as the Sylvia Lawry data set, all patients can also be on active treatment in a clinical trial. However, historical control groups can have very different outcomes with the same inclusion criteria. For example, patients in different secondary progressive trials of interferon beta-1b had very different disease progression results, although patients were selected with the same inclusion criteria. Active comparator trials are another option in which one treatment is compared with an existing treatment.[2] For example, the Betaferon Efficacy Yielding Outcomes of a New Dose (BEYOND) trial compares 2 doses of interferon beta-1b with glatiramer.
Criteria to ethically perform placebo trials in MS patients must start with informed consent, including the option of being randomized to not receive treatment. Patients should not have tolerated, failed, or refused current treatments. The trial design should be of short duration and have surrogate markers. Rules for patients to drop out of the trial for worsening disease should be clearly delineated. Equipoise is paramount in which the investigator is uncertain of the outcomes before placing patients on placebo.[1]
An important outcomes measure of the pivotal phase 3 MS trials has been confirmed progression of disability. Patients are considered to have confirmed progression with a documented Extended Disability Status Scale (EDSS) increase of greater than 1.0 point on 2 examinations 3-6 months apart. Butzkueven and colleagues[3] assessed whether the trial definition of "confirmed disability progression" equates with "long-term sustained" EDSS progression with the prospectively acquired global MSBase dataset. In their analysis of 4043 relapsing-remitting and secondary progressive patients from 33 contributing MS clinics, 981 had confirmed progression of disability on neurologic exams at least 3 months apart. After 2 years of confirmed disability progression in relapsing-remitting disease, 25% of patients' EDSS scores returned to baseline. After 5 years, 50% of relapsing-remitting patients with confirmed progression returned to baseline level of disability. Fifteen percent of patients with secondary progressive disease returned to baseline level of disability.
Early evaluation of new treatments frequently relies on MRI endpoints. Many trials, such as the 20-mg vs 40-mg glatiramer acetate phase 2 trial, have screened patients for enhancing lesions to assist in determining efficacy with fewer patients. Zhao and colleagues[4] examined the short-term changes in monthly gadolinium-enhancing lesion activity in patients with different initial activity levels. In the 65 placebo patients studied, 49% had no enhancing lesions at baseline. Of this group, 75% developed new lesions over 9 months. All patients with enhancing lesions at baseline had at least 2 more enhancing lesions over 9 months with monthly MRI scans.
Can The Weather Make You Sick?
"WE TALK ABOUT FLU SEASON, SPRING FEVER, WINTER DOLDRUMS, SUMMER COLDS....WE'RE TOO HOT, TOO COLD, TOO HUMID (AND SOMETIMES IN OCTOBER...THE WEATHER IS JUST RIGHT). BUT FOR SOME, THE WEATHER IS PART OF THEIR ILLNESS. METEOROLOGIST JERE HOUGH INTRODUCES YOU TO PEOPLE WHO ALWAYS WATCH THE FORECAST WITH SPECIAL INTEREST.
Laurie Setterstrom was diagnosed with Multiple Sclerosis about ten years ago...and like others with MS, hot weather is the enemy.
She laughs,?"I need to be moving to Alaska...that's where I need to be."
The problem is so pronounced that all kinds of devices have been developed for MS sufferers to help them keep cool.
A battery-powered cooler collar that cools the blood as it passes through the neck. There are wraps and even a vest that cool by evaporation. She holds up blue vest, "You have to soak it in cold water for ten or fifteen minutes."
Setterstrom has?figured out a way to get an outside chore done...like cleaning her car, "People think I'm crazy but I like to do it late at night."
Ironically, Laurie Setterstrom used to love lying in the hot Gulf Coast sun."
"WE TALK ABOUT FLU SEASON, SPRING FEVER, WINTER DOLDRUMS, SUMMER COLDS....WE'RE TOO HOT, TOO COLD, TOO HUMID (AND SOMETIMES IN OCTOBER...THE WEATHER IS JUST RIGHT). BUT FOR SOME, THE WEATHER IS PART OF THEIR ILLNESS. METEOROLOGIST JERE HOUGH INTRODUCES YOU TO PEOPLE WHO ALWAYS WATCH THE FORECAST WITH SPECIAL INTEREST.
Laurie Setterstrom was diagnosed with Multiple Sclerosis about ten years ago...and like others with MS, hot weather is the enemy.
She laughs,?"I need to be moving to Alaska...that's where I need to be."
The problem is so pronounced that all kinds of devices have been developed for MS sufferers to help them keep cool.
A battery-powered cooler collar that cools the blood as it passes through the neck. There are wraps and even a vest that cool by evaporation. She holds up blue vest, "You have to soak it in cold water for ten or fifteen minutes."
Setterstrom has?figured out a way to get an outside chore done...like cleaning her car, "People think I'm crazy but I like to do it late at night."
Ironically, Laurie Setterstrom used to love lying in the hot Gulf Coast sun."
MCT-125 human trial: MultiCell requests permission for MS trial or fatigue
MultiCell Technologies has said that it will seek authorization to proceed with its phase IIb MCT-125 human trial for fatigue in multiple sclerosis in the UK.
MCT-125 has already completed a phase II clinical trial and demonstrated significant efficacy in reducing chronic fatigue in multiple sclerosis patients. There is no drug specifically approved for the treatment of chronic fatigue in multiple sclerosis patients.MORE
MultiCell Technologies has said that it will seek authorization to proceed with its phase IIb MCT-125 human trial for fatigue in multiple sclerosis in the UK.
MCT-125 has already completed a phase II clinical trial and demonstrated significant efficacy in reducing chronic fatigue in multiple sclerosis patients. There is no drug specifically approved for the treatment of chronic fatigue in multiple sclerosis patients.MORE
NEW PATENT: METHOD FOR DIAGNOSING MULTIPLE SCLEROSIS
Abstract Disclosed is a method for diagnosing multiple sclerosis and more
particularly to a method for diagnosing multiple sclerosis by measuring levels
of antibodies to glycans in a biological sample.
Abstract Disclosed is a method for diagnosing multiple sclerosis and more
particularly to a method for diagnosing multiple sclerosis by measuring levels
of antibodies to glycans in a biological sample.
Tuesday, November 21, 2006
Using water to battle MS, [By COURTNEY KLEMM -- Herald & Review]
DECATUR - Colorful air-filled rubber balls splashed in the water with each attempt to pass them to the next person.
Laughter and constant chatter echoed against the walls of the aquatics area at the Greater Decatur Y as the women gathered in the pool passed the gertie exercise balls to their right, then to their left, then over their heads.
The gertie balls allow muscular stretching that is always beneficial but provides even more health advantages to those with multiple sclerosis.
In the very first YMCA aquatics class geared toward people with multiple sclerosis, class director Barb Entrot leads participants each Tuesday morning through a program designed to help them with health problems associated with MS.
Entrot attended an aquatics training workshop for multiple sclerosis in Chicago to aid in creating a strategy for the class. Each session varies, but all are composed of a warm-up, stretching, range-of-motion exercises, such as shoulder raises and wrist rolls, and a cardio or endurance portion, usually including jumping jacks and leg lifts in the pool. Resistant floats and the side of the pool provide means to work each muscle.
"We try to cover all of the body parts," Entrot said. "It helps with their balance and well-being and helps them to accomplish movement."
And after five weeks in the class, the participants said they have noticed a difference in both their bodies and the emotional well-being they receive from the support and light conversation that consumes each session.
"I just want to be in the best health that I can, to get fit and feel healthier as much as I can," said Sue Durham. "It's given me the motivation to keep moving at home and has helped with the pain I have with my hip on the right side. You have to keep your muscles limber and stretch. You need to do it every day, whether here or at home."
Each day is different when it comes to the pain associated with multiple sclerosis, so Entrot makes sure to check on her participants.
"Sometimes they might have a hard time getting dressed, and other days, no problem at all," she said. "I have to watch them closely, and I'm always asking if they're OK."
With the water in the pool kept at a pleasant 83 degrees, the class participants are able to comfortably work their muscles and joints.
"I find myself looking forward to coming to the Y and getting in the water," said Carla Andrick, who attends the class with her mother, Virginia Wilhelm, whom she calls the "lucky one" to drive her, since Andrick is no longer able. "Water exercise is the best thing for MS. Everything you can't do on land, you can do in the water."
Water aerobics allows people with multiple sclerosis to move various parts of their body they wouldn't normally be able to while being easier on their joints, Entrot said.
Carolyn Mack said she has always turned to exercise to improve the health effects of multiple sclerosis and wanted to attend the aquatics program to continue working out and be a source of encouragement to others.
"I do regular aerobics three times a week, and I wanted to do this to motivate other people to do it," she said.
As each class comes to an end, Entrot leads the participants in a cool down, with stretching to relieve any muscle tension from the session. In balletlike moves, the members of the group raise their arms, and with their hands over their heads, they clap a round of applause for the step toward better health they have taken in a fight against the disease that plagues their bodies.
MORE -- Herald & Review]
DECATUR - Colorful air-filled rubber balls splashed in the water with each attempt to pass them to the next person.
Laughter and constant chatter echoed against the walls of the aquatics area at the Greater Decatur Y as the women gathered in the pool passed the gertie exercise balls to their right, then to their left, then over their heads.
The gertie balls allow muscular stretching that is always beneficial but provides even more health advantages to those with multiple sclerosis.
In the very first YMCA aquatics class geared toward people with multiple sclerosis, class director Barb Entrot leads participants each Tuesday morning through a program designed to help them with health problems associated with MS.
Entrot attended an aquatics training workshop for multiple sclerosis in Chicago to aid in creating a strategy for the class. Each session varies, but all are composed of a warm-up, stretching, range-of-motion exercises, such as shoulder raises and wrist rolls, and a cardio or endurance portion, usually including jumping jacks and leg lifts in the pool. Resistant floats and the side of the pool provide means to work each muscle.
"We try to cover all of the body parts," Entrot said. "It helps with their balance and well-being and helps them to accomplish movement."
And after five weeks in the class, the participants said they have noticed a difference in both their bodies and the emotional well-being they receive from the support and light conversation that consumes each session.
"I just want to be in the best health that I can, to get fit and feel healthier as much as I can," said Sue Durham. "It's given me the motivation to keep moving at home and has helped with the pain I have with my hip on the right side. You have to keep your muscles limber and stretch. You need to do it every day, whether here or at home."
Each day is different when it comes to the pain associated with multiple sclerosis, so Entrot makes sure to check on her participants.
"Sometimes they might have a hard time getting dressed, and other days, no problem at all," she said. "I have to watch them closely, and I'm always asking if they're OK."
With the water in the pool kept at a pleasant 83 degrees, the class participants are able to comfortably work their muscles and joints.
"I find myself looking forward to coming to the Y and getting in the water," said Carla Andrick, who attends the class with her mother, Virginia Wilhelm, whom she calls the "lucky one" to drive her, since Andrick is no longer able. "Water exercise is the best thing for MS. Everything you can't do on land, you can do in the water."
Water aerobics allows people with multiple sclerosis to move various parts of their body they wouldn't normally be able to while being easier on their joints, Entrot said.
Carolyn Mack said she has always turned to exercise to improve the health effects of multiple sclerosis and wanted to attend the aquatics program to continue working out and be a source of encouragement to others.
"I do regular aerobics three times a week, and I wanted to do this to motivate other people to do it," she said.
As each class comes to an end, Entrot leads the participants in a cool down, with stretching to relieve any muscle tension from the session. In balletlike moves, the members of the group raise their arms, and with their hands over their heads, they clap a round of applause for the step toward better health they have taken in a fight against the disease that plagues their bodies.
MORE -- Herald & Review]
Stada to abandon Interferon-beta project for multiple sclerosis - Forbes.com
FRANKFURT (AFX) - Stada Arzneimittel AG said it is no longer to pursue its Interferon-beta project to treat multiple sclerosis, as limited marketing opportunities did not justify completion of the project.
The company said that the project, which was launched in 2001, did not have a completion date or a potential peak sales figure.
The move is part of the company's reorganisation of its Biosimilar projects.
Stada announced that it is now focusing on its Epo-zeta and Filgrastim projects.
The company is to transfer development, manufacturing and distribution rights for Epo-zeta for the US and Canada to US-based competitor Hospira.MORE - Forbes.com
FRANKFURT (AFX) - Stada Arzneimittel AG said it is no longer to pursue its Interferon-beta project to treat multiple sclerosis, as limited marketing opportunities did not justify completion of the project.
The company said that the project, which was launched in 2001, did not have a completion date or a potential peak sales figure.
The move is part of the company's reorganisation of its Biosimilar projects.
Stada announced that it is now focusing on its Epo-zeta and Filgrastim projects.
The company is to transfer development, manufacturing and distribution rights for Epo-zeta for the US and Canada to US-based competitor Hospira.MORE - Forbes.com
Adult Stem Cells Give Hope for Healing - FOXNews.com - MORE
Stem cell research is a much debated and controversial topic in modern medicine. However, the controversy around embryonic stem cells has also sparked an interest in a less ethically risky option: using stem cells taken from adults to treat various diseases.
To understand the amazing impact stem cell use can have for modern medicine you should first understand what a stem cell is.
At first, all life comes from a single cell. Humans grow from just one fertilized egg into a tremendously complex organism. Each organ has its own specialized cells that "know" how to carry out their own special functions. A stem cell, however, is like that very first cell we all originally come from: it is able to become any cell we need it to be.
In fact, the fertilized egg is basically our very first stem cell; from it, we develop all the other cell types found within the human body. Adults still have stem cells in their tissues as they grow and adult stem cells have been collected from bone marrow for many years. They are different from embryonic stem cells because they have passed a certain point of "no return,"meaning they cannot develop into an organ; however the possibilities are still tremendous as they can become different cell types.
Adult stem cells can be found in the umbilical cord blood and placenta when babies are born, and can be saved or "banked" to offer new parents a certain peace of mind. Although stem cells from cord blood are the child's "own," and therefore can be transplanted without the risk of rejection, there is a limit to the number of cells that can be acquired.
"It can save a child's life if certain blood disorders develop in the early years, but there may not be enough cells to treat an older child," Dr. Guerra continued. "Adult stem cells could hold the key to life-long health by facilitating treatment of devastating diseases and as a result increasing longevity."
Banked adult stem cells can be used at a future date to treat a wide array of diseases, such as heart disease, osteoporosis, arthritis, and many other diseases. Because these are a person's own cells, there is no concern about finding a matched donor.
"A great benefit to using one's own adult stem cells is the fact that you do not have to worry about rejection of cells since your own cells are used for your treatment," noted Neostem's CEO and Chairman Dr. Robin Smith, M.D., M.B.A.
To place this in perspective, consider that less than 20 percent of patients who need a bone marrow transplant actually find a match in time to treat their disease.
There are currently over 160 clinical trials using autologous stem cells. New studies describing the clinical benefits of adult stem cells in the treatment of diseases are being published almost daily. Countless research teams around the world are trying to study the possibility of using adult stem cells to grow skin, improve muscle, build cartilage, and regenerate the vital cells of a failing organ.
In addition, growing interest in regenerative medicine is also driving the demand for convenient stem cell banking methods. The results so far are definitely promising.
-- The Journal of American Medical Association reported a study where 50 percent of patients with Lupus (SLE) treated with stem cells were disease-free five years after treatment.
-- The Journal of Rheumatology showed that 73 percent of individuals with rheumatoid arthritis were able to be controlled on medication after being treated with stem cells.
-- The journal Nature even reported the use of adult stem cells to repair vision of blind mice.
We will likely continue to see more results of clinical trials using adult stem cells to address other diseases such as diabetes, wound healing and multiple sclerosis, to name a few. If these techniques are successful, we would have much less need for donor organs; we would improve conditions that previously decreased someone's lifespan or quality of life, such as diabetes, blindness, Parkinson's, Alzheimer's; multiple sclerosis could be cured.
According to Dr. Guerra, clinical trials are already under way in the cardiovascular department:
"As far as treatments go, great advances are being made in improving cardiac status of those individuals with end-stage heart disease and repairing the damaged tissue of those having heart attacks," Dr. Guerra said. "Additionally, you do not have the potential issue of tumor formation which has been seen with embryonic cells," he added.
Now adults have the option to collect and save their own stem cells through a non-invasive and safe procedure, a bio-insurance for future use, and one that may just save your life.
Dr. Manny Alvarez is the managing editor of health news at Foxnews.com, and is a regular medical contributor on the FOX News Channel. He is chairman of the Department of Obstetrics and Gynecology and Reproductive Science at Hackensack University Medical Center in New Jersey. Additionally, Alvarez is Adjunct Professor of Obstetrics and Gynecology at New York University School of Medicine in New York City.
Adult Stem Cells Give Hope for Healing - FOXNews.com - MORE
Stem cell research is a much debated and controversial topic in modern medicine. However, the controversy around embryonic stem cells has also sparked an interest in a less ethically risky option: using stem cells taken from adults to treat various diseases.
To understand the amazing impact stem cell use can have for modern medicine you should first understand what a stem cell is.
At first, all life comes from a single cell. Humans grow from just one fertilized egg into a tremendously complex organism. Each organ has its own specialized cells that "know" how to carry out their own special functions. A stem cell, however, is like that very first cell we all originally come from: it is able to become any cell we need it to be.
In fact, the fertilized egg is basically our very first stem cell; from it, we develop all the other cell types found within the human body. Adults still have stem cells in their tissues as they grow and adult stem cells have been collected from bone marrow for many years. They are different from embryonic stem cells because they have passed a certain point of "no return,"meaning they cannot develop into an organ; however the possibilities are still tremendous as they can become different cell types.
Adult stem cells can be found in the umbilical cord blood and placenta when babies are born, and can be saved or "banked" to offer new parents a certain peace of mind. Although stem cells from cord blood are the child's "own," and therefore can be transplanted without the risk of rejection, there is a limit to the number of cells that can be acquired.
"It can save a child's life if certain blood disorders develop in the early years, but there may not be enough cells to treat an older child," Dr. Guerra continued. "Adult stem cells could hold the key to life-long health by facilitating treatment of devastating diseases and as a result increasing longevity."
Banked adult stem cells can be used at a future date to treat a wide array of diseases, such as heart disease, osteoporosis, arthritis, and many other diseases. Because these are a person's own cells, there is no concern about finding a matched donor.
"A great benefit to using one's own adult stem cells is the fact that you do not have to worry about rejection of cells since your own cells are used for your treatment," noted Neostem's CEO and Chairman Dr. Robin Smith, M.D., M.B.A.
To place this in perspective, consider that less than 20 percent of patients who need a bone marrow transplant actually find a match in time to treat their disease.
There are currently over 160 clinical trials using autologous stem cells. New studies describing the clinical benefits of adult stem cells in the treatment of diseases are being published almost daily. Countless research teams around the world are trying to study the possibility of using adult stem cells to grow skin, improve muscle, build cartilage, and regenerate the vital cells of a failing organ.
In addition, growing interest in regenerative medicine is also driving the demand for convenient stem cell banking methods. The results so far are definitely promising.
-- The Journal of American Medical Association reported a study where 50 percent of patients with Lupus (SLE) treated with stem cells were disease-free five years after treatment.
-- The Journal of Rheumatology showed that 73 percent of individuals with rheumatoid arthritis were able to be controlled on medication after being treated with stem cells.
-- The journal Nature even reported the use of adult stem cells to repair vision of blind mice.
We will likely continue to see more results of clinical trials using adult stem cells to address other diseases such as diabetes, wound healing and multiple sclerosis, to name a few. If these techniques are successful, we would have much less need for donor organs; we would improve conditions that previously decreased someone's lifespan or quality of life, such as diabetes, blindness, Parkinson's, Alzheimer's; multiple sclerosis could be cured.
According to Dr. Guerra, clinical trials are already under way in the cardiovascular department:
"As far as treatments go, great advances are being made in improving cardiac status of those individuals with end-stage heart disease and repairing the damaged tissue of those having heart attacks," Dr. Guerra said. "Additionally, you do not have the potential issue of tumor formation which has been seen with embryonic cells," he added.
Now adults have the option to collect and save their own stem cells through a non-invasive and safe procedure, a bio-insurance for future use, and one that may just save your life.
Dr. Manny Alvarez is the managing editor of health news at Foxnews.com, and is a regular medical contributor on the FOX News Channel. He is chairman of the Department of Obstetrics and Gynecology and Reproductive Science at Hackensack University Medical Center in New Jersey. Additionally, Alvarez is Adjunct Professor of Obstetrics and Gynecology at New York University School of Medicine in New York City.
Adult Stem Cells Give Hope for Healing - FOXNews.com - MORE
REGIONAL RESULTS OF MS STUDY TO BE RELEASED PAW PAW - In response to people's concern about a perceived high number of multiple sclerosis cases in small Illinois communities, a study has been completed.
Its findings comparing local incidence of the disease to that nationwide will be presented at 7 p.m. Dec. 5 at the Community Building on Main Street.
Health Systems Research of the University of Illinois College of Medicine at Rockford submitted a grant to study the prevalence of the disease in the towns of DePue, Lewistown, Morrison, Paw Paw and Savanna.
Presentations will be held in each of the five study communities.
The grant was received in October 2002 from the Agency for Toxic Substances and Disease Registry, which is a division of the Centers for Disease Control and Prevention.
Five areas in the nation were funded to study MS and ALS (Lou Gehrig's disease).
Health Systems Research sought to find all individuals with MS and ALS who had lived in the ZIP code areas of the five targeted communities from 1998 to 2002.
Study subjects then completed information on their medical, residential and occupational history and gave permission for their medical records to be reviewed to verify the diagnosis.
For more information, contact Joel Cowen, principal investigator, at (800) 854-4461 or joelc@uic.edu.
Its findings comparing local incidence of the disease to that nationwide will be presented at 7 p.m. Dec. 5 at the Community Building on Main Street.
Health Systems Research of the University of Illinois College of Medicine at Rockford submitted a grant to study the prevalence of the disease in the towns of DePue, Lewistown, Morrison, Paw Paw and Savanna.
Presentations will be held in each of the five study communities.
The grant was received in October 2002 from the Agency for Toxic Substances and Disease Registry, which is a division of the Centers for Disease Control and Prevention.
Five areas in the nation were funded to study MS and ALS (Lou Gehrig's disease).
Health Systems Research sought to find all individuals with MS and ALS who had lived in the ZIP code areas of the five targeted communities from 1998 to 2002.
Study subjects then completed information on their medical, residential and occupational history and gave permission for their medical records to be reviewed to verify the diagnosis.
For more information, contact Joel Cowen, principal investigator, at (800) 854-4461 or joelc@uic.edu.

house accommodates MS [Daily Nebraskan]
Five years ago, when Cecilia Rossiter was diagnosed with multiple sclerosis, a disease of the central nervous system that affects mobility, vision and muscles, she knew she needed a new beginning away from the big city anonymity and extreme housing costs.
So Rossiter, a professional cellist originally from Omaha then living in Washington, D. C., decided to return to Nebraska and was attracted by the tight-nit university community in Lincoln.
But she struggled to find a house that accommodated her disabilities until she heard about a collaborative program between the University of Nebraska-Lincoln College of Architecture and NeighborWorks Lincoln, a group that promotes homeownership, which brought her dream house to life.
Mark Hoistad, the director of the UNL architecture program, said the collaborative effort allowed graduate architecture students to design the house, provide the construction documents and frame the building while NeighborWorks hired a contractor to finish the construction of the house.
The project was designed in the fall of 2004 by a final proposal out of a class of 15 students with construction not finalized until the summer of 2005.
Hoistad, who taught the design class that produced the house's final design, said the house was designed to conform to American with Disabilities Act standards to make it accessible to anyone with a mobility disability.
After living in the house for a few months, Rossiter said she appreciates the house's built-in features, such as a ramp in the garage and three-foot-wide doors, because she is beginning to have mobility problems.
Those with disabilities often feel trapped in buildings that aren't accessible, she said.
Hoistad said other features of the house were a garden lot that cut into the frame of the house and a glass corridor area that used passive solar energy.
"The corridor allows sun in the winter and shade in the summer," Hoistad said.
The corridor's cinderblock wall absorbed heat in the summer and keeps heat from the basement inside during the winter, Rossiter said.
She also said the energy-efficient corridor served another purpose.
"It's lovely in an unintended way," Rossiter said. "I have low vision. The bank of windows makes me feel much happier with sunlight coming in."MORE: UNL-designed house accommodates MS
Monday, November 20, 2006
"Drug combo fuels hope for MS: Patients in small study showed shocking reversal of symptoms" - MSNBC.com
LONDON - Four years ago, 28-year-old multiple sclerosis patient Karen Ayres was wheelchair-bound and paralyzed. "I was trapped in a body that wouldn't do anything," she says. Now, following an experimental drug treatment, she has regained mobility and is studying for a doctoral degree.
Ayres was one of 27 patients with aggressive MS who was treated in an open trial with a course of cancer-drug mitoxantrone and copaxone, which is used to treat relapsing MS.
Like Ayres, many of the other patients in the study experienced results so remarkable that some MS experts, while expressing caution, are now taking a second look at the preliminary experiment.
A three-year controlled study is being launched at 10 centers across the United Kingdom to further investigate the potential of the drug combination. The results of the initial trial, led by Dr. Mike Boggild at the Walton Centre in Liverpool, will be published next month in the Journal of Neurology.
Mitoxantrone is an anti-cancer drug so powerful that it is potentially toxic and can only be used safely in the short term. So, Boggild and his colleagues combined its use with copaxone — a notoriously slow-acting drug.
"We decided to overlap the treatments because we wanted to give some time to copaxone to build up its effect," says Boggild.
What happened next was dramatic. "Patients who were just the worst of the worst did remarkably well," Boggild said.
"We think we've tapped into an unexpected synergy between the two drugs that gives you more than the sum of the parts," he says. With a few exceptions, Boggild says most of the patients treated with the drug combination are now essentially "trouble-free."
Though one patient developed acute leukemia — a known side effect to mitoxantrone treatment — Boggild says the majority of patients haven't had disease relapses.MORE: "Drug combo fuels hope for MS: Patients in small study showed shocking reversal of symptoms" - MSNBC.com
LONDON - Four years ago, 28-year-old multiple sclerosis patient Karen Ayres was wheelchair-bound and paralyzed. "I was trapped in a body that wouldn't do anything," she says. Now, following an experimental drug treatment, she has regained mobility and is studying for a doctoral degree.
Ayres was one of 27 patients with aggressive MS who was treated in an open trial with a course of cancer-drug mitoxantrone and copaxone, which is used to treat relapsing MS.
Like Ayres, many of the other patients in the study experienced results so remarkable that some MS experts, while expressing caution, are now taking a second look at the preliminary experiment.
A three-year controlled study is being launched at 10 centers across the United Kingdom to further investigate the potential of the drug combination. The results of the initial trial, led by Dr. Mike Boggild at the Walton Centre in Liverpool, will be published next month in the Journal of Neurology.
Mitoxantrone is an anti-cancer drug so powerful that it is potentially toxic and can only be used safely in the short term. So, Boggild and his colleagues combined its use with copaxone — a notoriously slow-acting drug.
"We decided to overlap the treatments because we wanted to give some time to copaxone to build up its effect," says Boggild.
What happened next was dramatic. "Patients who were just the worst of the worst did remarkably well," Boggild said.
"We think we've tapped into an unexpected synergy between the two drugs that gives you more than the sum of the parts," he says. With a few exceptions, Boggild says most of the patients treated with the drug combination are now essentially "trouble-free."
Though one patient developed acute leukemia — a known side effect to mitoxantrone treatment — Boggild says the majority of patients haven't had disease relapses.MORE: "Drug combo fuels hope for MS: Patients in small study showed shocking reversal of symptoms" - MSNBC.com
"Multiple sclerosis a complex, but common, problem" [Scranton Times-Tribune 11/20/2006] : "Judging by my e-mail inquiries, it%u2019s fairly safe to say that most readers of this column either directly or indirectly know someone who has multiple sclerosis.
MS has been a part of my life — as a physical therapist, friend and relative of some incredible people and their families affected by this disease.
According to the National Multiple Sclerosis Society, MS affects about 400,000 people in the United States and is second only to trauma as the most common cause of neurological disability for those in early to middle adulthood.
MS is almost three times as common in women, and it’s very uncommon before adolescence or after 50. But the risk increases from the teen years until 50.
Multiple sclerosis is considered an autoimmune disease. The body’s immune system does not work properly; it fails to attack foreign substances such as bacteria. Instead, the system allows the body to attack normal tissues and create diseases such as MS, rheumatoid arthritis and lupus.
In MS, the immune system attacks the brain and spinal cord of the central nervous system. Each nerve has an outer covering of a fatty material — myelin — for insulation to improve the transmission and conductivity of impulses or messages to and from the brain. Damage to the myelin interrupts the ability of messages to travel through the spinal cord and to other areas of the body, such as the muscles in the arms and legs.
Due to this “short circuiting,” the brain becomes unable to send or receive messages. In MS, scar tissue or plaque (sclerosis) replaces the fatty myelin in “multiple” areas. This is also called demyelination.
Symptoms: The symptoms associated with MS vary greatly. The amount, frequency and speed of the demyelination process are directly related to the loss of strength and function in daily activities.MORE:
MS has been a part of my life — as a physical therapist, friend and relative of some incredible people and their families affected by this disease.
According to the National Multiple Sclerosis Society, MS affects about 400,000 people in the United States and is second only to trauma as the most common cause of neurological disability for those in early to middle adulthood.
MS is almost three times as common in women, and it’s very uncommon before adolescence or after 50. But the risk increases from the teen years until 50.
Multiple sclerosis is considered an autoimmune disease. The body’s immune system does not work properly; it fails to attack foreign substances such as bacteria. Instead, the system allows the body to attack normal tissues and create diseases such as MS, rheumatoid arthritis and lupus.
In MS, the immune system attacks the brain and spinal cord of the central nervous system. Each nerve has an outer covering of a fatty material — myelin — for insulation to improve the transmission and conductivity of impulses or messages to and from the brain. Damage to the myelin interrupts the ability of messages to travel through the spinal cord and to other areas of the body, such as the muscles in the arms and legs.
Due to this “short circuiting,” the brain becomes unable to send or receive messages. In MS, scar tissue or plaque (sclerosis) replaces the fatty myelin in “multiple” areas. This is also called demyelination.
Symptoms: The symptoms associated with MS vary greatly. The amount, frequency and speed of the demyelination process are directly related to the loss of strength and function in daily activities.MORE:
"Multiple sclerosis a complex, but common, problem" [Scranton Times-Tribune 11/20/2006] : "Judging by my e-mail inquiries, it%u2019s fairly safe to say that most readers of this column either directly or indirectly know someone who has multiple sclerosis.
MS has been a part of my life — as a physical therapist, friend and relative of some incredible people and their families affected by this disease.
According to the National Multiple Sclerosis Society, MS affects about 400,000 people in the United States and is second only to trauma as the most common cause of neurological disability for those in early to middle adulthood.
MS is almost three times as common in women, and it’s very uncommon before adolescence or after 50. But the risk increases from the teen years until 50.
Multiple sclerosis is considered an autoimmune disease. The body’s immune system does not work properly; it fails to attack foreign substances such as bacteria. Instead, the system allows the body to attack normal tissues and create diseases such as MS, rheumatoid arthritis and lupus.
In MS, the immune system attacks the brain and spinal cord of the central nervous system. Each nerve has an outer covering of a fatty material — myelin — for insulation to improve the transmission and conductivity of impulses or messages to and from the brain. Damage to the myelin interrupts the ability of messages to travel through the spinal cord and to other areas of the body, such as the muscles in the arms and legs.
Due to this “short circuiting,” the brain becomes unable to send or receive messages. In MS, scar tissue or plaque (sclerosis) replaces the fatty myelin in “multiple” areas. This is also called demyelination.
Symptoms: The symptoms associated with MS vary greatly. The amount, frequency and speed of the demyelination process are directly related to the loss of strength and function in daily activities.MORE:
MS has been a part of my life — as a physical therapist, friend and relative of some incredible people and their families affected by this disease.
According to the National Multiple Sclerosis Society, MS affects about 400,000 people in the United States and is second only to trauma as the most common cause of neurological disability for those in early to middle adulthood.
MS is almost three times as common in women, and it’s very uncommon before adolescence or after 50. But the risk increases from the teen years until 50.
Multiple sclerosis is considered an autoimmune disease. The body’s immune system does not work properly; it fails to attack foreign substances such as bacteria. Instead, the system allows the body to attack normal tissues and create diseases such as MS, rheumatoid arthritis and lupus.
In MS, the immune system attacks the brain and spinal cord of the central nervous system. Each nerve has an outer covering of a fatty material — myelin — for insulation to improve the transmission and conductivity of impulses or messages to and from the brain. Damage to the myelin interrupts the ability of messages to travel through the spinal cord and to other areas of the body, such as the muscles in the arms and legs.
Due to this “short circuiting,” the brain becomes unable to send or receive messages. In MS, scar tissue or plaque (sclerosis) replaces the fatty myelin in “multiple” areas. This is also called demyelination.
Symptoms: The symptoms associated with MS vary greatly. The amount, frequency and speed of the demyelination process are directly related to the loss of strength and function in daily activities.MORE:
Type 1 Diabetes Ups MS Risk [MORE: Physician's Weekly Article-November 20, 2006 Vol. XXIII, No. 44]
People with type 1 diabetes appear to be three times more likely than others to develop multiple sclerosis (MS), according to a study published in the July 2006 Archives of Neurology. A group of researchers assessed the link between type 1 diabetes and MS by looking at two population-based disease registers: the Danish Hospital Discharge Register and the Danish Multiple Sclerosis Register. The study revealed that first-degree relatives of patients with MS had a 63% increased risk of developing type 1 diabetes. The authors concluded that their study “demonstrates an intra-individual and, to a lesser degree, an intra-familial co-occurrence of multiple sclerosis and type 1 diabetes.
People with type 1 diabetes appear to be three times more likely than others to develop multiple sclerosis (MS), according to a study published in the July 2006 Archives of Neurology. A group of researchers assessed the link between type 1 diabetes and MS by looking at two population-based disease registers: the Danish Hospital Discharge Register and the Danish Multiple Sclerosis Register. The study revealed that first-degree relatives of patients with MS had a 63% increased risk of developing type 1 diabetes. The authors concluded that their study “demonstrates an intra-individual and, to a lesser degree, an intra-familial co-occurrence of multiple sclerosis and type 1 diabetes.
Repair and Protection: National MS Society' "Targeted Research: and Protection Initiative"
What if we could actually reverse the damage that MS causes, restoring function to those who have been living with the disease for years? Recent scientific advances in many different fields are now, for the first time, coming together to bring the dream of protecting and repairing brain tissue and restoring function within our grasp. The Society now targets this vital quest with a program that could dramatically impact future disease management, improve the lives of the estimated two million people worldwide living with MS, and could ultimately lead to a cure.
A specially convened National MS Society Task Force determined that the best way to accelerate nerve tissue repair is to bring together the appropriate clinical specialists and basic laboratory scientists to form partnerships to conduct all elements of the study, from basic research to human clinical trials.
Collaborating to make tissue repair and protection a reality
To ensure the speediest results, the task force recommended that the Society fund as many centers as can meet the extensive expectations ofMORE: Repair and Protection: National MS Society' "Targeted Research: and Protection Initiative"
What if we could actually reverse the damage that MS causes, restoring function to those who have been living with the disease for years? Recent scientific advances in many different fields are now, for the first time, coming together to bring the dream of protecting and repairing brain tissue and restoring function within our grasp. The Society now targets this vital quest with a program that could dramatically impact future disease management, improve the lives of the estimated two million people worldwide living with MS, and could ultimately lead to a cure.
A specially convened National MS Society Task Force determined that the best way to accelerate nerve tissue repair is to bring together the appropriate clinical specialists and basic laboratory scientists to form partnerships to conduct all elements of the study, from basic research to human clinical trials.
Collaborating to make tissue repair and protection a reality
To ensure the speediest results, the task force recommended that the Society fund as many centers as can meet the extensive expectations ofMORE: Repair and Protection: National MS Society' "Targeted Research: and Protection Initiative"
MCT-125: New drug for the treatment of chronic fatigue in multiple sclerosis patients
MultiCell Technologies, Inc. (OTCBB:MCET), developing first-in-class drugs based on advanced immune system modulation and other proprietary technologies, today announced that it will seek allowance to proceed from the Medicines and Healthcare Products Regulatory Agency (“MHRA”) in the UK for its planned Phase IIb MCT-125 human clinical trial for the treatment of chronic fatigue in multiple sclerosis patients.
MultiCell has scheduled a meeting with the MHRA for December 1, 2006 to present the Company’s clinical development plan for MCT-125. Additionally, the Company will request that the MHRA allow the Company to proceed to enroll patients in a proposed Phase IIb clinical trial to determine the minimum efficacious therapeutic dose for MCT-125.
“We are hopeful MCT-125 will become an important therapy available to the millions of patients suffering from Primary Multiple Sclerosis-related Fatigue (PMSF),” said Dr. Stephen M. Chang, Chief Executive Officer of MultiCell. “The purpose of the proposed Phase IIb dose-ranging trial is to determine the minimum efficacious dose for MCT-125 prior to commencing a pivotal Phase III trial. The initial Phase II trial conducted by Amarin only determined a single efficacious dose, and we need to know a range over which MCT-125 will remain effective,” noted Dr. Chang.
MCT-125 targets fatigue associated with MS, an autoimmune disease in which immune cells attack and destroy the myelin sheath protecting neurons in the brain and spinal cord. About two million people worldwide are afflicted with MS, and approximately 70 percent of them report fatigue as the worst symptom of their disease.
MultiCell’s therapeutic pipeline includes drug candidates some of which are in various advanced stages of human clinical trials. These therapies include:
* MCT-125 for the treatment of chronic fatigue in MS patients. MCT-125 completed a Phase II clinical trial and demonstrated significant efficacy in reducing chronic fatigue in MS patients. There is no drug specifically approved for the treatment of chronic fatigue in MS patients anywhere in the world.
* MCT-175 for the treatment of relapsing-remitting MS. MCT-175, in preclinical development for the treatment of relapsing-remitting MS, targets disease specific autoaggressive T-cells that destroy the myelin sheath of nerve cells. MCT-175 successfully ameliorated the disease in animal models. MORE ON: MCT-125
MultiCell Technologies, Inc. (OTCBB:MCET), developing first-in-class drugs based on advanced immune system modulation and other proprietary technologies, today announced that it will seek allowance to proceed from the Medicines and Healthcare Products Regulatory Agency (“MHRA”) in the UK for its planned Phase IIb MCT-125 human clinical trial for the treatment of chronic fatigue in multiple sclerosis patients.
MultiCell has scheduled a meeting with the MHRA for December 1, 2006 to present the Company’s clinical development plan for MCT-125. Additionally, the Company will request that the MHRA allow the Company to proceed to enroll patients in a proposed Phase IIb clinical trial to determine the minimum efficacious therapeutic dose for MCT-125.
“We are hopeful MCT-125 will become an important therapy available to the millions of patients suffering from Primary Multiple Sclerosis-related Fatigue (PMSF),” said Dr. Stephen M. Chang, Chief Executive Officer of MultiCell. “The purpose of the proposed Phase IIb dose-ranging trial is to determine the minimum efficacious dose for MCT-125 prior to commencing a pivotal Phase III trial. The initial Phase II trial conducted by Amarin only determined a single efficacious dose, and we need to know a range over which MCT-125 will remain effective,” noted Dr. Chang.
MCT-125 targets fatigue associated with MS, an autoimmune disease in which immune cells attack and destroy the myelin sheath protecting neurons in the brain and spinal cord. About two million people worldwide are afflicted with MS, and approximately 70 percent of them report fatigue as the worst symptom of their disease.
MultiCell’s therapeutic pipeline includes drug candidates some of which are in various advanced stages of human clinical trials. These therapies include:
* MCT-125 for the treatment of chronic fatigue in MS patients. MCT-125 completed a Phase II clinical trial and demonstrated significant efficacy in reducing chronic fatigue in MS patients. There is no drug specifically approved for the treatment of chronic fatigue in MS patients anywhere in the world.
* MCT-175 for the treatment of relapsing-remitting MS. MCT-175, in preclinical development for the treatment of relapsing-remitting MS, targets disease specific autoaggressive T-cells that destroy the myelin sheath of nerve cells. MCT-175 successfully ameliorated the disease in animal models. MORE ON: MCT-125
Sunday, November 19, 2006
Rolling Back the Ravages of MS
What if we could actually reverse the damage that MS causes, restoring function to those who have been living with the disease for years? Recent scientific advances in many different fields are now, for the first time, coming together to bring the dream of protecting and repairing brain tissue and restoring function within our grasp. The Society now targets this vital quest with a program that could dramatically impact future disease management, improve the lives of the estimated two million people worldwide living with MS, and could ultimately lead to a cure.
A specially convened National MS Society Task Force determined that the best way to accelerate nerve tissue repair is to bring together the appropriate clinical specialists and basic laboratory scientists to form partnerships to conduct all elements of the study, from basic research to human clinical trials.
Collaborating to make tissue repair and protection a reality
To ensure the speediest results, the task force recommended that the Society fund as many centers as can meet the extensive expectations ofMORE
Educating Patients for Pharmaceutical Companies [NurseZone]
By Debra Wood, RN, contributor
With physicians pressed for time to teach patients, pharmaceutical companies have stepped up to fill the void, sending expertly trained nurses into medical offices and patients’ homes armed with the knowledge to ease patients’ fears and improve their adherence with treatment plans, which should lead to better outcomes.
“Patients benefit tremendously in learning more about their disease process,” said Rosanne Burson, RN, MSN, CDE, field manager in Detroit for HELP (Hands on Education for Lantus/Apidra Patients), a program administered by Innovex Inc. for sanofi-aventis U.S. LLC. “Many times we are picking up issues where they weren’t using something properly, or they were afraid.”.....
The nurses provide no hands-on care. In the MS LifeLines (www.mslifelines.com) program for Serono Inc. and Pfizer Inc, nurses educate patients about multiple sclerosis, teach them how to self-administer Rebif, an interferon beta 1-a for relapsing forms of MS, and coach them through the injection process. Teaching takes place in the patients’ home and begins with a 1.5-hour to 2-hour session.
“I feel like I’m helping them get over this hurdle, especially the newly diagnosed patients,” said Mary Ann Caron, RN, BSN, MSCN, an MS LifeLines nurse educator in Washington, D.C. “Some patients are more emotional about it.”
The nurses visit when the clinician starts the patient on Rebif, again two months later and at 11 months. In between visits, the nurses communicate with patients by telephone. Patients also can access information through the MS LifeLines nurse-staffed call center.
“In addition to developing and maintaining long-term relationships with my patients with phone calls and visits with my patients, another rewarding aspect of my job is being able to help patients give their own injection,” Caron said. “This is a huge step for patients to undertake as this means that they are able to take control of their disease and, hopefully, take control of their future.”
Caron recently taught a gentleman to self-administer Rebif after he had been returning to the doctor’s office for years, frightened to give it himself. With much encouragement, Caron taught him the skill and now he enjoys greater freedom.
Corrie Westwood, RN, BSN, MSCN, another MS LifeLines nurse, was diagnosed with MS five years ago and completed a National Multiple Sclerosis Society nursing fellowship, after learning she had the disease. The former labor and delivery nurse moved to Arizona from Colorado to work for Innovex in this program. She has no regrets.
“There’s no time limit on it,” said Westwood, explaining what she likes about the job. “We can sit and talk. Usually I go over whatever questions they have, and then I go over the disease process, the treatments and what to expect from that, and managing side effects.”
Westwood believes having MS helps her with most patients. She often tells them and serves as an inspiration that they too may be able to live a full, productive life.
“I understand a little bit more what they are going through,” she said. “Sometimes I am the only person they know who has MS.”
However, she usually refrains from sharing the information with patients who have progressed and are suffering from cognitive or mobility problems.MORE
By Debra Wood, RN, contributor
With physicians pressed for time to teach patients, pharmaceutical companies have stepped up to fill the void, sending expertly trained nurses into medical offices and patients’ homes armed with the knowledge to ease patients’ fears and improve their adherence with treatment plans, which should lead to better outcomes.
“Patients benefit tremendously in learning more about their disease process,” said Rosanne Burson, RN, MSN, CDE, field manager in Detroit for HELP (Hands on Education for Lantus/Apidra Patients), a program administered by Innovex Inc. for sanofi-aventis U.S. LLC. “Many times we are picking up issues where they weren’t using something properly, or they were afraid.”.....
The nurses provide no hands-on care. In the MS LifeLines (www.mslifelines.com) program for Serono Inc. and Pfizer Inc, nurses educate patients about multiple sclerosis, teach them how to self-administer Rebif, an interferon beta 1-a for relapsing forms of MS, and coach them through the injection process. Teaching takes place in the patients’ home and begins with a 1.5-hour to 2-hour session.
“I feel like I’m helping them get over this hurdle, especially the newly diagnosed patients,” said Mary Ann Caron, RN, BSN, MSCN, an MS LifeLines nurse educator in Washington, D.C. “Some patients are more emotional about it.”
The nurses visit when the clinician starts the patient on Rebif, again two months later and at 11 months. In between visits, the nurses communicate with patients by telephone. Patients also can access information through the MS LifeLines nurse-staffed call center.
“In addition to developing and maintaining long-term relationships with my patients with phone calls and visits with my patients, another rewarding aspect of my job is being able to help patients give their own injection,” Caron said. “This is a huge step for patients to undertake as this means that they are able to take control of their disease and, hopefully, take control of their future.”
Caron recently taught a gentleman to self-administer Rebif after he had been returning to the doctor’s office for years, frightened to give it himself. With much encouragement, Caron taught him the skill and now he enjoys greater freedom.
Corrie Westwood, RN, BSN, MSCN, another MS LifeLines nurse, was diagnosed with MS five years ago and completed a National Multiple Sclerosis Society nursing fellowship, after learning she had the disease. The former labor and delivery nurse moved to Arizona from Colorado to work for Innovex in this program. She has no regrets.
“There’s no time limit on it,” said Westwood, explaining what she likes about the job. “We can sit and talk. Usually I go over whatever questions they have, and then I go over the disease process, the treatments and what to expect from that, and managing side effects.”
Westwood believes having MS helps her with most patients. She often tells them and serves as an inspiration that they too may be able to live a full, productive life.
“I understand a little bit more what they are going through,” she said. “Sometimes I am the only person they know who has MS.”
However, she usually refrains from sharing the information with patients who have progressed and are suffering from cognitive or mobility problems.MORE








